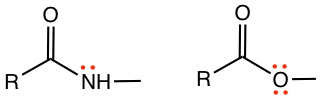Chapter 5: More Reactions on Aromatic Compounds
5.2 Directing Effects of the Substituents on EAS
A substituent on the benzene ring also influences the orientation that the incoming EAS takes place (i.e., which position the incoming group occupies), this is the directing effect. There are two types of directors specifically: ortho-para directors and meta directors.
5.2.1 Two Types of Directors: Ortho-para Directors or Meta Directors
If the substituent on the benzene ring is an ortho-para director, the incoming group will be directed (oriented) to the ortho- and para-position of the director itself.
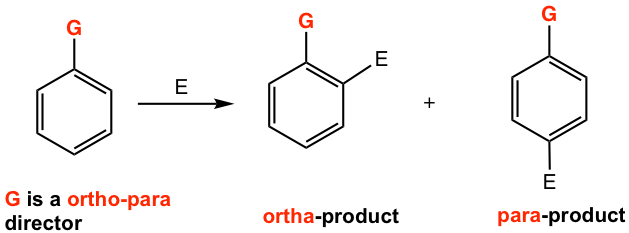
If the substituent on the benzene ring is a meta director, the incoming group will be directed (oriented) to the meta-position of the director itself.

The directing effect of a substituent is closely related to the activating-deactivating effect, specifically:
- Activating groups are ortho-para directors;
- Deactivating groups (except halogens) are meta directors;
- Halogen substituents are weakly deactivating groups, and are ortho-para directors.
In this section, we will take a closer look at the directing effects of different groups, from the experimental results, to compare and get the trending. The explanations for why the directing effect works in a certain way will be discussed in the next section 5.2.3.
Let’s start with some experimental results.
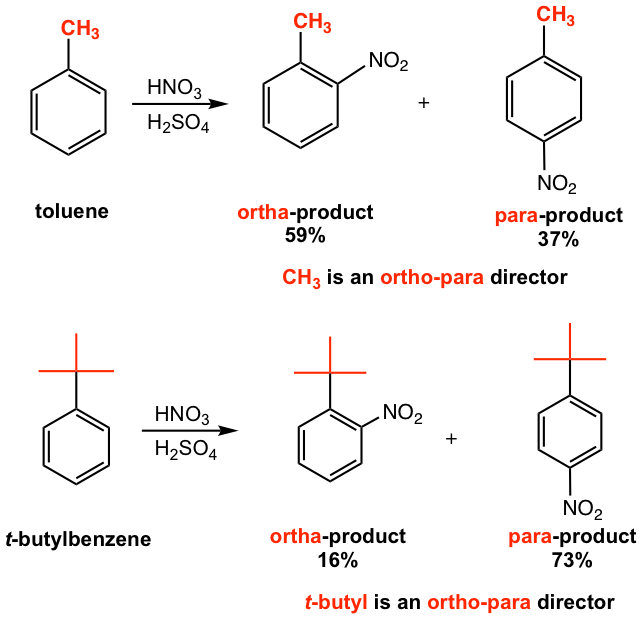
The experimental results (Fig. 5.1h) for the nitration reaction of toluene and t-butylbenzene clearly indicate that alkyl groups are ortho-para directors, the combination of ortho- and para-products account for at least 90% of the products for both reactions. Comparing the two reactions further, however, we will see the different ortho/para product ratios. For toluene, substituent CH3 is a small size group, the ortho/para ratio is about 2:1, and it matches the statistical ratio of ortho and para positions. When the size of the substituent increases to t-butyl, para-product is the major one. The steric effect can explain this, that the ortho position is less favorable because of the steric hindrance for the bulky t-butyl group. Usually, the bulky alkyl group can be regarded as a para director predominantly.
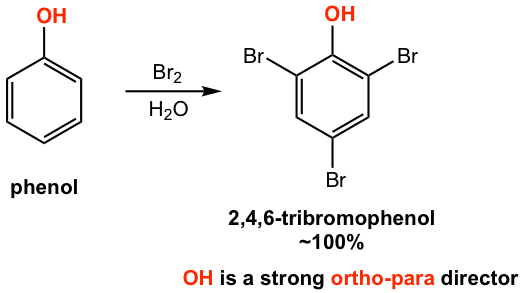
The reaction condition and result of the bromination of phenol (Fig. 5.2d) might be surprising to us. Lewis acid catalyst, for example, FeBr3, is usually required for bromination. However, no catalyst is required for the bromination of phenol, the reaction occurs readily in a bromine solution, with 2,4,6-tribromophenol obtained as a product. All the ortho and para positions are brominated in the product. This result indicates that OH is a strong activating group and strong ortho-para director. Generally, substituents with lone pair electrons on the atom adjacent to the benzene ring, for example, -OH, -OR, -NH3, -NH2R, are stronger activating groups than groups without lone pair electrons (like the alkyl group), and these strong activating groups are strong ortho-para directors.
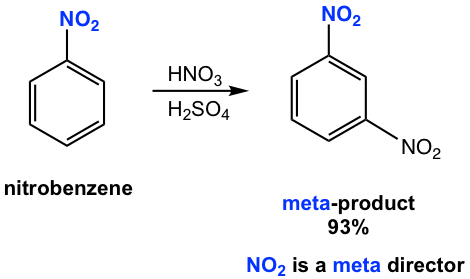
The Nitro group is a strong deactivating group, with a reaction rate about 1/10000 times that of benzene. Most deactivating groups are meta directors, and the nitration of nitrobenzene takes place in the meta position with a 93% yield (Fig. 5.2e).
5.2.2 Unique Halogen Substituent: Deactivating Ortho-Para Director
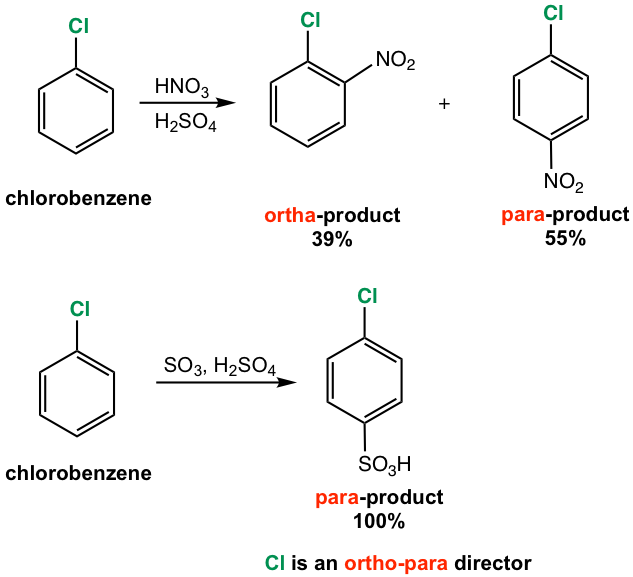
Halogen substituents (Cl, Br, and I) affect the EAS in a rather unique way. Halobenzene undergoes aromatic substitution much slower than benzene, which indicates halogen is the deactivating group. However, the incoming group is oriented on ortho- and para-positions predominantly, as the reaction results of chlorobenzene shown in Fig. 5.2f. The halogen substituent is therefore a deactivating ortho-para director.
In summary, different substituents can be classified into several categories, depending on the directing effect, and the strength of activating/deactivating ability, which is shown in Table 5.1.
| Ortho-para directors | Meta directors |
| strongly activating
|
strongly deactivating
-NO2, -NH3+, -NR3+, -CF3
|
| moderately activating
|
moderately deactivating
|
| weakly activating
-R (alkyl), -Ar (aryl) |
|
| weakly deactivating
-F, -Cl, -Br, -I |
Table 5.1 Effect of substituents on EAS reaction
Notes for Table 5.1:
- Substituents with lone-pair electrons on the atom bonded directly on the benzene ring, such as -OH, -OR, -NH3, -NH2R, etc, are strong activating groups and strong ortho-para directors.
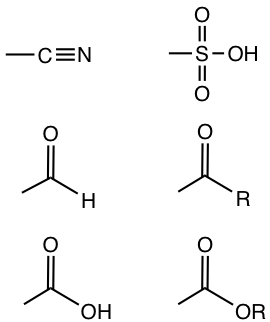
- Substituents containing multiple bonds besides the atom with lone-pair electrons are moderate activating groups. The electron-withdrawing effect of the multiple bonds weakens the electron-donating effect of the lone-pair electron. Examples include -OCOR, -NHCOR.
- Alkyl groups (no lone pair electrons) are weak activating groups.
- Halogens are weakly deactivating groups, but ortho-para directors.
- Substituents with heteroatoms (N, O, etc) that don’t have lone-pair electrons, for example, NH
3+, only the inductive electron-withdrawing effect applies because of the high electronegativity of the heteroatom, these substituents are strong deactivating groups. - Substituents with multiple bonds connected directly on the benzene ring, for example, -COR, -COOR, -C≡N, are generally moderate deactivating groups.
5.2.3 Explanation of Directing (Orientation) Effect: Understand the Reasons Behind
Ortho-para Directors
The directing effect of a substituent can be explained from two perspectives.
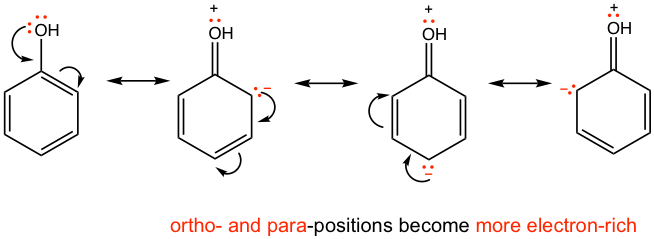
The first explanation focuses on how the substituent affects the electron density of the benzene ring, and it is a more straightforward way to understand. For the electron-donating group, such as -OH, the electron density is transferred to the benzene ring via the resonance effect. From the resonance structures shown below in Fig. 5.2g, it is clear that the extra electron density is localized on the ortho and para carbons, therefore these carbons are more likely to react with the electrophile.
The second way of explanation relies on the stability comparison of the arenium ion intermediates, that would arise from the attack on different positions.

For ortho and para attacks of phenol, there are four resonance structures available for the arenium ion, and one of them shows special stability with all atoms having complete octets (Fig. 5.2h). Because of the special stability of this resonance structure, it makes a large contribution to the hybrid structure, and stabilizes the arenium ion significantly. For meta attack, however, only three resonance structures are available, and none of the resonance structures has all complete octets. These observations suggest that the ortho- and para-substituted arenium ions are considerably more stable than those from the meta attack, as a result, these are the preferred pathway for the reaction.
Meta Directors
Most deactivating groups are meta directors, this can also be explained by two perspectives. Only the first way is shown here to simplify the process.
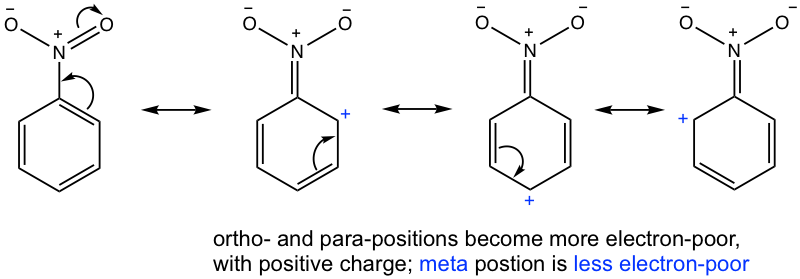
The electron-withdrawing effect via resonance makes the ortho- and para-positions most electron-poor, therefore meta position is a “better” choice for electrophilic attack since it is relatively more electron-rich than ortho and para positions.
Understand the Unusual Halogens
Although most deactivating groups are meta directors, halogens are different. Halogens are weakly deactivating groups, however, they are also ortho-para directors. The seems contradictory behavior can be explained that the reactivity of halobenzene is affected by the electron-withdrawing inductive effect (because of the electronegativity of halogen), and the orientation effect is determined by the electron-donating resonance effect (by lone pair electrons on halogen).
The electron-donating via resonance effect requires orbital overlapping between halogen and carbon on the benzene ring. The relatively large size of halogens (Cl, Br, and I) makes such overlapping less effective, so the inductive effect wins over the resonance effect for changing the electron density on the benzene ring. The halobenzene is slightly more electron-poor than benzene, so the halogen substituent is the weakly deactivating group.
The orientation effect of halogen can be explained through the different stability of arenium ions by the attack on different positions, in a similar way as for the OH group.
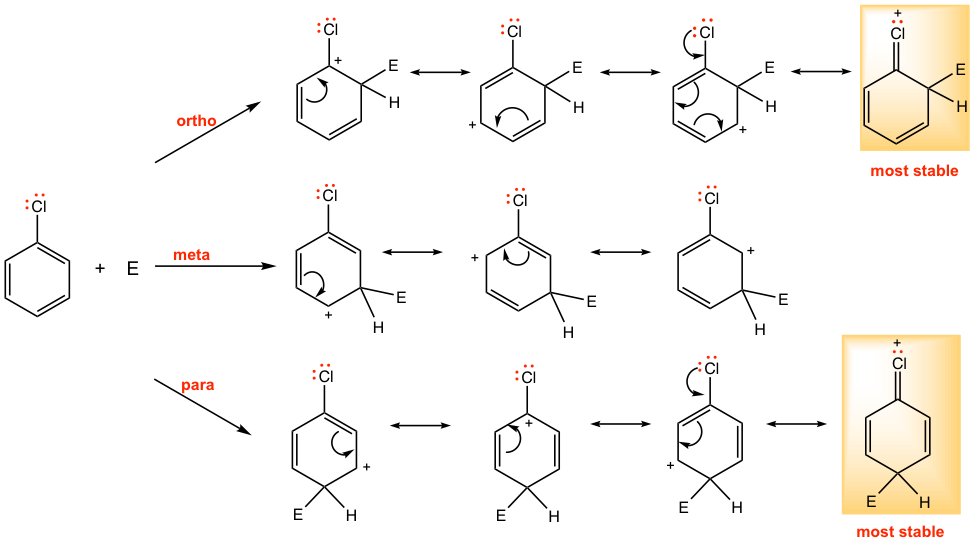
As shown in Fig. 5.2j, for chlorobenzene, there is one more resonance structure with a complete octet available for the arenium ion intermediate in ortho and para attack, the arenium ion is stabilized significantly. For meta attack, however, only three resonance structures are available, and none of the resonance structures has all complete octets. Therefore the ortho- and para-substituted arenium ions are considerably more stable than those from the meta attack, and these are the preferred pathways for the reaction.