Chapter 4: Aromatic Compounds
4.5 Electrophilic Aromatic Substitution (EAS) of Aromatic Compounds
Reactivities of aromatic compounds arise from their electronic properties, that is the aromaticity (or special stability) of these compounds. To retain the special stability, benzene does not undergo addition reactions like other alkene. Instead, substitution is the most common reaction for benzene and other aromatic compounds.

In a substitution reaction, one or more hydrogens on benzene can be replaced by a variety of different groups, for example, halogen, alkyl group, or carbonyl group, this provides the chance to produce huge numbers of different derivates of benzene. Since the benzene ring is very electron-rich (with six π electrons), it attracts electrophile and electrophilic aromatic substitution (EAS) is the most common substitution mechanism for benzene.
Electrophilic Aromatic Substitution (EAS)
Electrophilic aromatic substitution of benzene is the reaction in which an electrophile replaces one of the hydrogen atoms of the benzene ring.
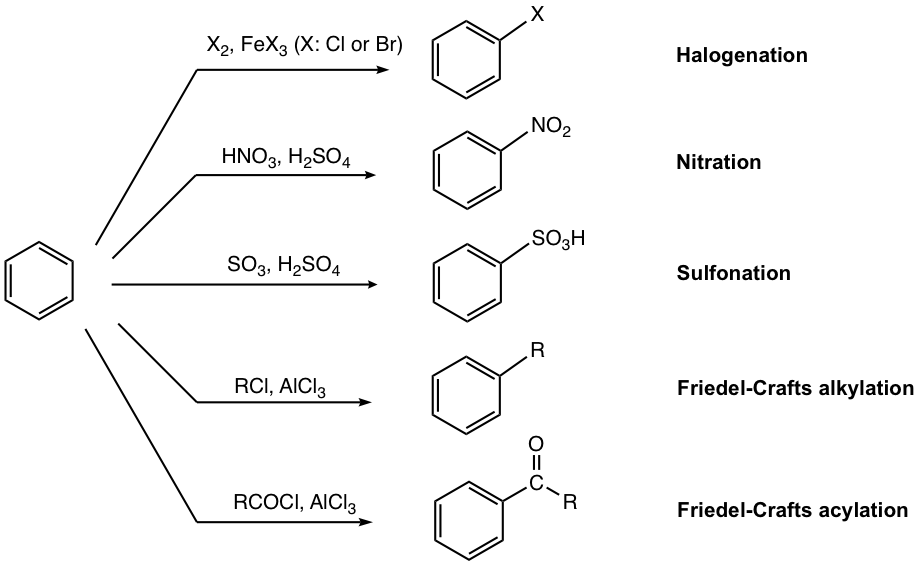
Depending on the group introduced onto the benzene ring, the reaction can also be named in a specific way, such as halogenation, nitration, etc. The five most common and useful EAS reactions are summarized in Fig. 4.5c. These reactions provide synthetic routes to a variety of benzene derivatives that have great applications in the synthesis of many other important compounds.
General Mechanism for EAS
The functional groups involved in different EAS reactions seem rather different, however, they all follow the same general mechanism. The difference only lies in the different structures of the electrophile for each type of EAS reaction. “E” is used to represent electrophiles in the general mechanism below (Fig. 4.5d).
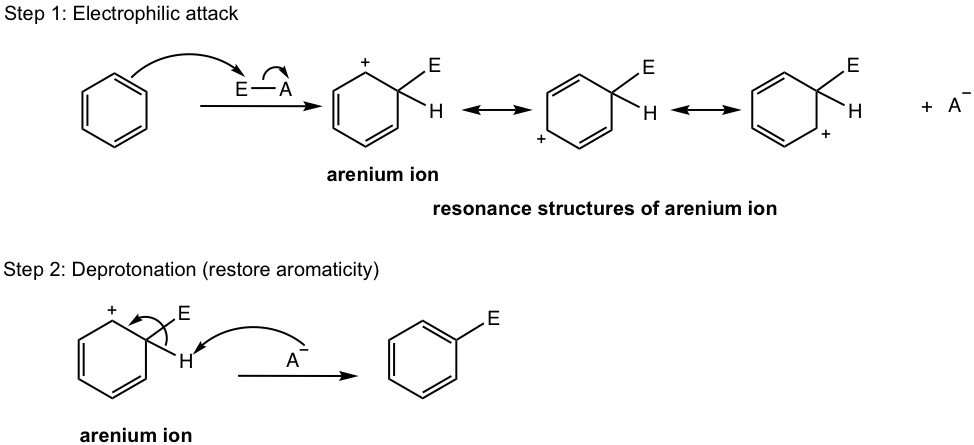
In the first step of the EAS, one electron pair of the benzene π system reacts with the electrophile, and this electron pair transfer forms a new σ bond. As a result, the electrophile is connected to one of the carbon atoms of the benzene ring, and a nonaromatic carbocation intermediate is formed which is called the arenium ion.
Formation of this arenium ion interrupts the aromatic system. Arenium ion, which is a cyclohexadienyl carbocation, has four sp2 hybridized carbons with a total of four π electrons, and it is nonaromatic. The carbocation intermediate is still stabilized by the resonance effect though, as shown in the mechanism.
In the 2nd step, a proton is removed from the carbon atom that bonds with the electrophile, and the aromaticity is restored in the substitution product.
In the following sections, we will examine the details of each type of EAS reaction. As mentioned earlier all the reactions follow the same general mechanism, the major differences are the structures of different electrophiles and the generation of the electrophiles.

