Chapter 4: Aromatic Compounds
4.4 Heterocyclic Aromatic Compounds
A cyclic compound that includes elements other than carbon is called a heterocyclic compound. The most commonly involved heteroatoms are nitrogen, oxygen, and sulfur. Some heterocyclic compounds are also aromatic and show special stability. Examples of heterocyclic aromatic compounds are given here.
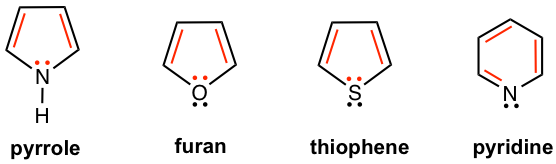
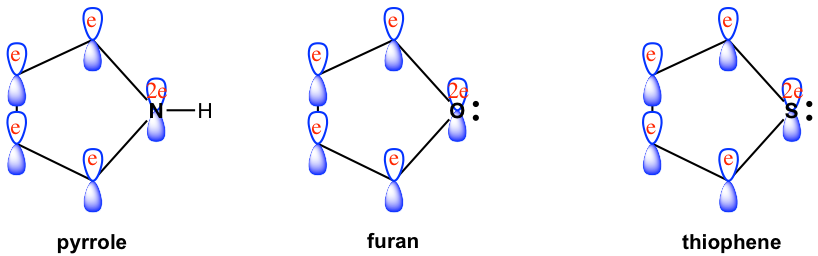
Among the above examples, pyrrole, furan, and thiophene share similar structural features. All hetero elements, N, O, and S respectively, are in sp2 hybridization. The unhybridized 2p orbital of N, O, and S holds one pair of lone-pair electrons, and the 2p orbital overlaps with the other four 2p orbitals of carbons (also in sp2 hybridization) and forms the conjugated system. The total number of π electrons (shown in red color in Fig. 4.4a) for the conjugated system therefore is 6, that is 2+4, which matches Huckel’s number and explains the aromaticity of the compounds.
The difference in the structure between pyrrole, furan, and thiophene is how the sp2 hybrid orbitals are used. For pyrrole, all three sp2 hybrid orbitals are involved in bonding (N in pyrrole has three single bonds), while for furan and thiophene only two of the sp2 hybrid orbitals are used for single bonds and the other sp2 orbital carries one lone-pair electron (shown in black color in Fig. 4.4a). The black color electron pair is not part of the aromatic system. The structural differences between the three heterocyclic aromatic compounds are highlighted in Fig. 4.4b.
Pyridine is a six-membered ring that is similar to a benzene ring, but with a nitrogen atom. All the elements in pyridine are sp2 hybridized, and the unhybridized 2p orbital for each element has one single electron that makes the total number of π electrons 6, the Huckel’s number for aromatic (shown in red color in Fig. 4.4a). Another structural difference between pyridine and benzene is that one of the sp2 orbitals of nitrogen holds one lone pair electron, while all the sp2 orbitals (for carbons) are used for bonding in benzene.
Pyrrole and Pyridine
Both pyrrole and pyridine are heterocyclic aromatic compounds with a nitrogen atom, and both nitrogen atoms have one pair of lone-pair electrons in the structure formula. Generally speaking, the nitrogen with lone pair electron should show basicity. It turns out that, however, pyridine is basic but pyrrole is not. How do you explain the difference in basicity between the two compounds?
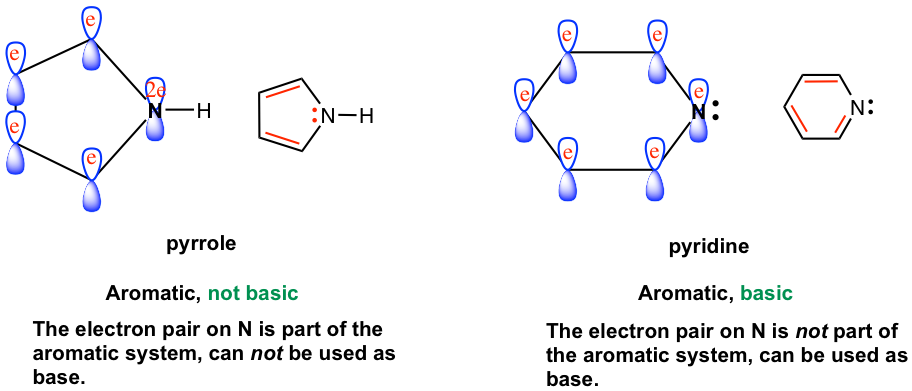
Such differences can be interpreted by taking a closer look at the structure of each (Fig. 4.4c). In pyridine, the lone pair electron (black color) is in the sp2 orbital of nitrogen and is not part of the aromatic system, therefore it can be used as a base. In pyrrole, the lone pair electron on nitrogen (red color) is in the unhybridized 2p orbital and is part of the aromatic system. Being part of the aromatic system prevents the electron pair from being used as a base, or not available for reacting with a proton. Thus pyrrole is not appreciably basic.
Example
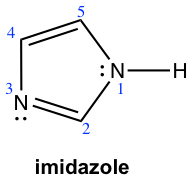
Imidazole has two nitrogens, only one is basic. Predict and explain which nitrogen atom is basic.
Answer:
The nitrogen atom in the #3 position is more basic, with the same reason for the basicity of pyrrole that the lone pair electron of that N is not part of the π system.

