Chapter 4: Aromatic Compounds
4.3 Hückel’s Rule: Aromatic, Anti-aromatic and Non-aromatic Species
The term “aromatic” is used to describe the compounds that also have the special stability as benzene does. The special stability comes from the unique electronic structure features of aromatic compounds. To determine whether the structure of a compound meets the requirement to be “aromatic”, Huckel’s rule can be applied.
Huckel’s rule, which was proposed by German chemist and physicist Erich Hückel in 1931, states that a cyclic, planar molecule has 4n+2 π electrons and is aromatic. In other words, the molecule must meet the following criteria to be an aromatic compound:
- cyclic (a ring structure)
- planar (all atoms in the molecule lie in the same plane)
- fully conjugate (each atom has a p orbital)
- there are 4n+2 π electrons (n=0 or any positive integer)
Other than aromatic compounds that have special stabilities, a compound could also be classified as nonaromatic or antiaromatic.
The cyclic compound that is not planar, or does not have all p orbitals fully conjugated, is nonaromatic. The nonaromatic compound is in the same energy level as the open-chain polyene with the same number of carbons.
The cyclic conjugated compound with 4n π electrons is antiaromatic. The antiaromatic compound is in a higher energy level than open-chain polyene with the same number of carbons, which means the antiaromatic compound has unusual instability.
Next, we will examine more cyclic structures by applying Huckel’s rule to determine which category a compound belongs to, aromatic, nonaromatic, or antiaromatic.
For the ring with 3, 5, or 7 carbons, there is always a sp3 hybridized carbon that does not have the unhybridized p orbital, so the ring is not fully conjugated and is nonaromatic.
Cyclobutadiene, the ring with 4 carbons, has 4 π electrons, so is antiaromatic.
Cyclooctatetraene, the eight-membered ring, also has 8 π electrons. However, it does not adopt the planar shape because of the strain, so it is nonaromatic.
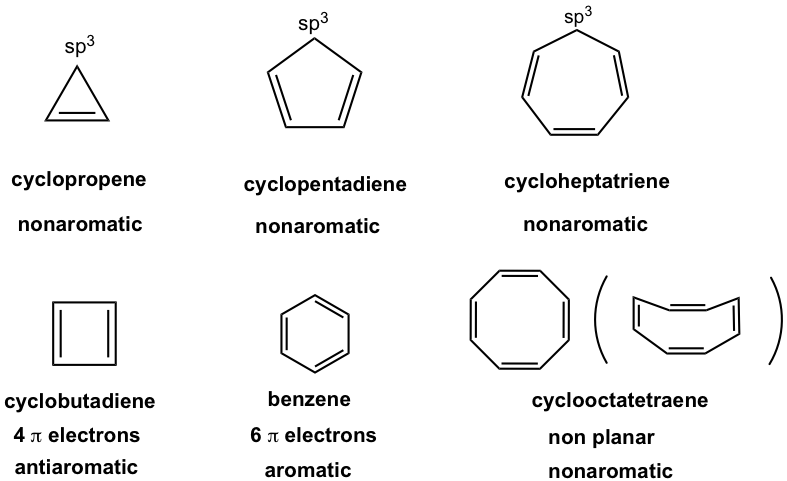
The properties of cyclopolyenes with three to eight carbons are summarized in Table 4.1.
| Annulene with an odd number of carbons | Annulene with an even number of carbons | ||||
| 3-carbon ring | 2 π electrons
non-conjugated |
nonaromatic | 4-carbon ring | 4 π electrons | antiaromatic |
|---|---|---|---|---|---|
| 5-carbon ring | 4 π electrons
non-conjugated |
nonaromatic | 6-carbon ring | 6 π electrons | aromatic |
| 7-carbon ring | 6 π electrons
non-conjugated |
nonaromatic | 8-carbon ring | 8 π electrons
non-planar |
nonaromatic |
Table. 4.1 Summary of cyclopolyenes properties with three to eight carbons (refer to Fig. 4.3a)
With the size of the ring increasing, the term annulene can be incorporated. Annulene is the general name for monocyclic compounds with a structure of alternating single and double bonds. The size of the ring is indicated by a number in the bracket. Benzene actually could be named [6] annulene, and cyclooctatetraene is [8]annulene.
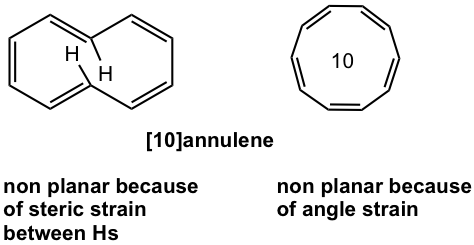
For [10]annulene, there are two possible structures. However, planar shape is not favored for any of them because of the strains. [10]annulene is nonaromatic.
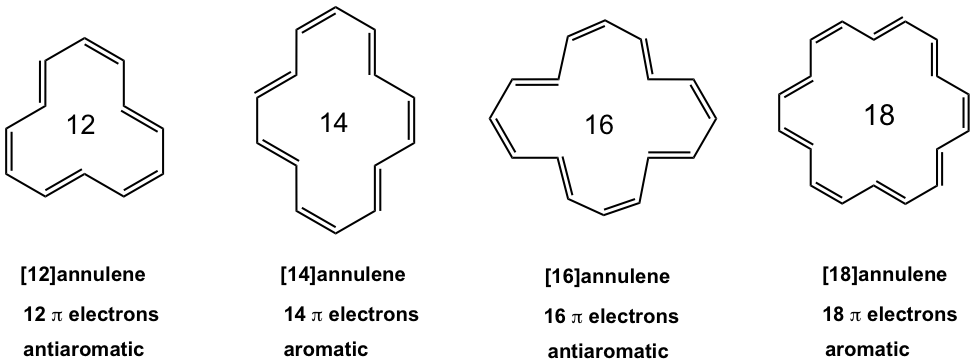
For annulenes with more carbons, the size of the ring is big enough to adopt a planar shape, without causing significant strains. So annulenes with 12 carbons or more are in planar shapes, and the property depends only on the total number of π electrons.
Polycyclic Aromatic Compounds
There are other types of aromatic compounds that contain more than one benzene rings. A representative type of such compound is polycyclic aromatic compounds (also called benzenoid aromatic compounds) that have two or more benzene rings fused together. The term fused means that two aromatic rings are connected by at least one common carbon-carbon bond. Some examples of polycyclic aromatic compounds are shown in Fig. 4.3d.
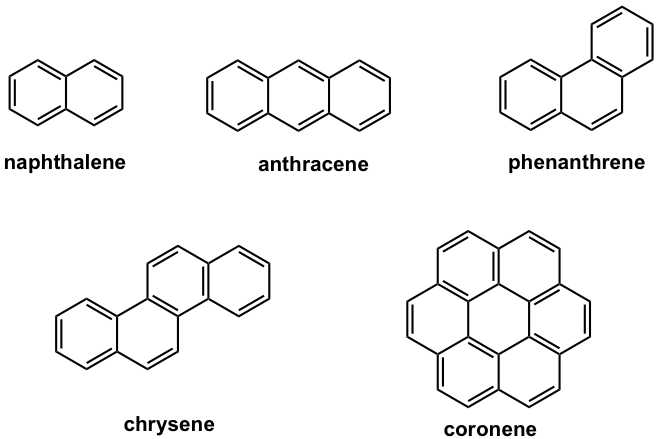
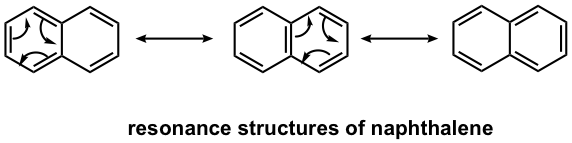
Naphthalene is a well-known polycyclic aromatic compound with two benzene rings fused together. As you may predict, the actual structure of naphthalene is a hybrid of three resonance structures, so it is highly stabilized by the resonance effect.
Aromaticity of Ions
Other than neutral molecules, some ionic species also show special stability due to aromaticity, and they are called aromatic ions. Huckel’s rule applies to ions as well for determining the aromaticity.
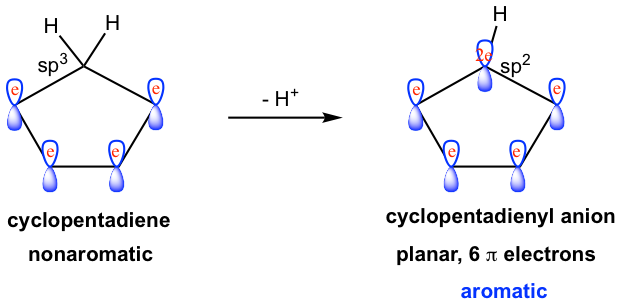
A typical example is the cyclopentadienyl anion. The neutral cyclopentadiene molecule is nonaromatic as we learned earlier, however, this molecule is unusually acidic as a hydrocarbon and has a pKa of about 16 (usually the pKa for allylic hydrogen is about 40). The strong acidity of the molecule indicates that the conjugate base, cyclopentadienyl anion, should be exceptionally stable. The fact of the unusual stability of cyclopentadienyl anion can be explained by the aromaticity of the anion.

After deprotonation, the initially sp3 hybridized carbon gains one lone pair election and becomes a sp2 hybridized carbon anion. The sp2 carbon therefore has an unhybridized 2p orbital that can overlap with all the other four 2p orbitals to form a conjugated system. Moreover, with the lone pair electron occupying the 2p orbital, there are a total of 6 π electrons in the system, that is 2 (the lone pair) + 4 (two double bonds). A planar ring with conjugated π orbitals, and 6 π electrons, the cyclopentadienyl anion is an aromatic ion. The aromaticity of the anion explains the unusual acidity of the cyclopentadiene molecule.