Chapter 3: Conjugated Unsaturated Systems
3.5 Diels-Alder Reaction
A special and superb reaction for conjugated diene is the Diels-Alder reaction, which is also called a 1,4-cycloaddition. In the Diels-Alder reaction, a conjugated diene, simply referred to as the diene, reacts with another reactant containing a double or triple bond called the dienophile (the species that likes the diene). During the reaction, two new sigma bonds formed that give a six-membered ring, together with a double bond in the ring that comes from the electron pair transfer.
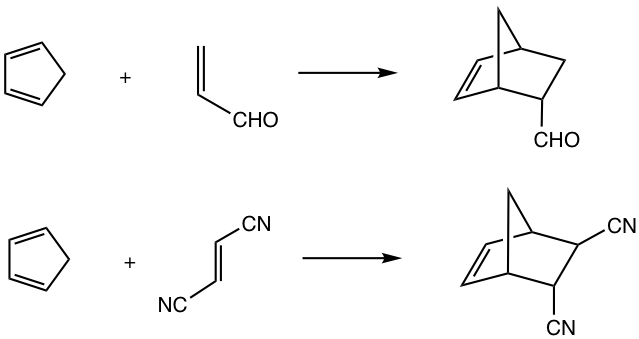
An example of the Diels-Alder Reaction is shown in Fig. 3.5a.

The Diels-Alder reaction offers a great way to build ring structure with great versatility, and has proved to be a synthesis tool with broad applications. Otto Diels and Kurt Alder were awarded the Nobel Prize in 1950 for this brilliant discovery.
3.5.1 Mechanism for Diels-Alder Reaction
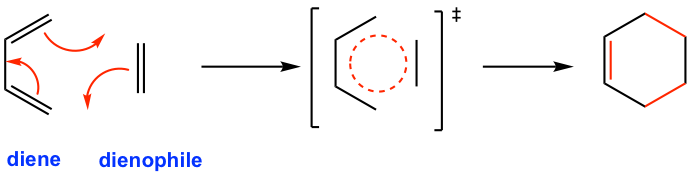
The mechanism for the Diels-Alder reaction (Fig. 3.5b) is a concerted process with all the three π electron pairs transfer taking place at the same time, through a cyclic transition state, to form the six-membered cyclic product. Cycloaddition reactions are classified according to the number of p electrons that interact. Since diene has four p electrons and the dienophile has two p electrons, the Diels-Alder reaction is a [4+2] cycloaddition reaction.
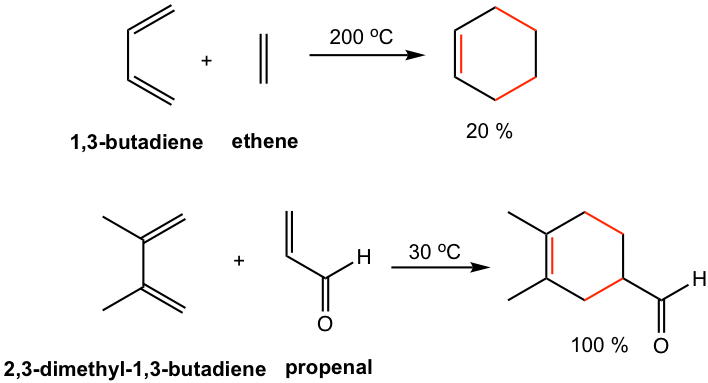
For the Diels-Alder reaction between simplest reactants, for example, 1,3-butadiene reacts and ethene, a high temperature is required and the product is obtained with a low yield. However, the reaction between 2,3-dimethyl-1,3-butadiene and propenal proceeds at 30 °C with an almost quantitative yield of the product, as shown in Fig. 3.5c. Let’s see next how the substituents on diene and dienophile affect the rate of the reaction.
3.5.2 Structure Requirements for Diene and Dienophile
The substituents on both reactants have significant impacts on the reaction speed.
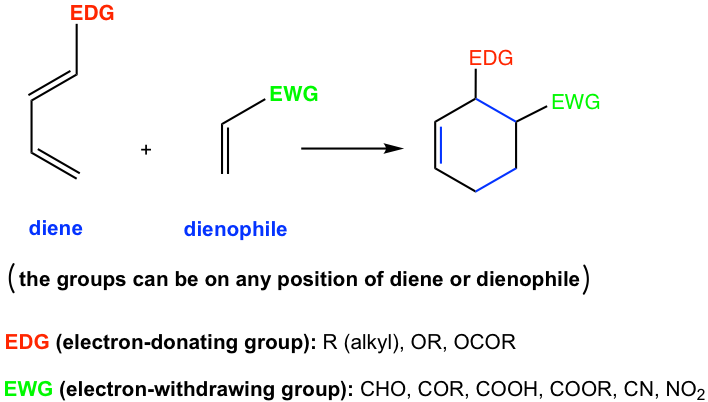
Generally, the Diels-Alder reaction is favored by the electron-donating groups in the dienes and the electron-withdrawing groups in the dienophiles.
Electron-donating groups include alkyl groups and groups that start with the heteroatom which has lone-pair electrons (for example O and N). The electron-withdrawing groups are generally groups with multiple bonds (for example C=O, N=O bonds etc). Examples of EDG and EWG are shown in Fig. 3.5d, more discussions on how to identify EDG and EWG are available in Chapter 5.
Dienes
As mentioned dienes with electron-donating groups are good candidates for Diels-Alder reactions, several examples of such dienes are shown in Fig. 3.5e.
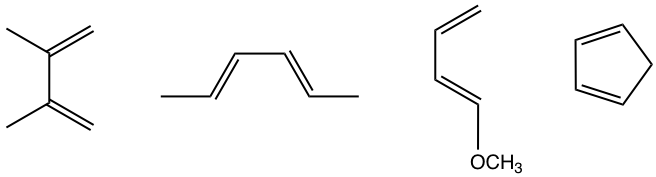
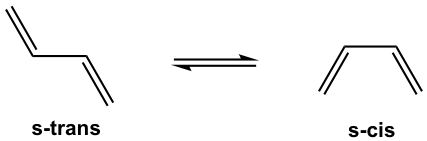
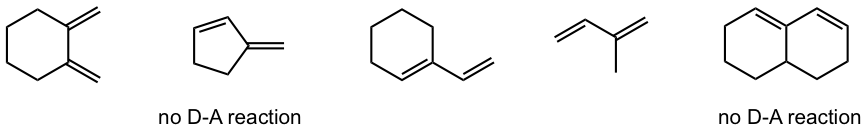
Since the Diels-Alder reaction is a concerted process, the diene must adopt an s-cis conformation for carbon atoms on both ends to bond simultaneously with the dienophile. Although the s-trans conformer is more stable for acyclic diene, the two are generally in rapid equilibrium (as shown in Fig. 3.5f), thus allowing the use of most acyclic diene in Diels-Alder reactions.
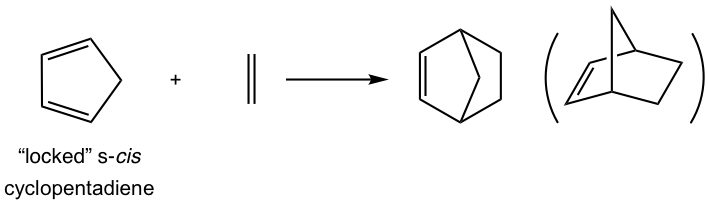
For cyclic dienes in which the double bonds are “locked” in the s-cis conformation, for example, cyclopentadiene, they are especially reactive for the Diels-Alder reaction. The reaction between cyclopentadiene and ethene gives a bicyclic product with a good yield.
Examples
Which of the following dienes cannot do the Diels-Alder reaction?
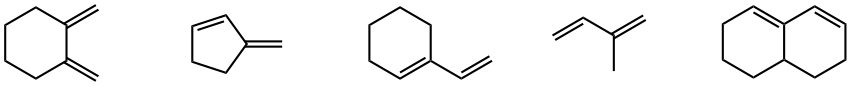
Answers:
Dienophiles
The electron-withdrawing groups on dienophiles speed up the Diels-Alder reaction. Several examples of dienophiles commonly applied are given below.
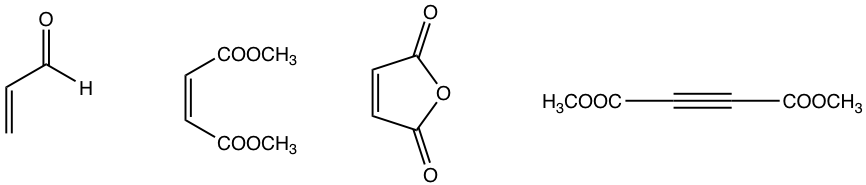
Examples
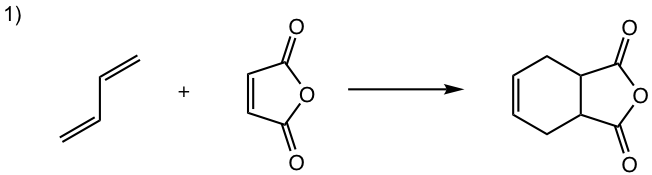
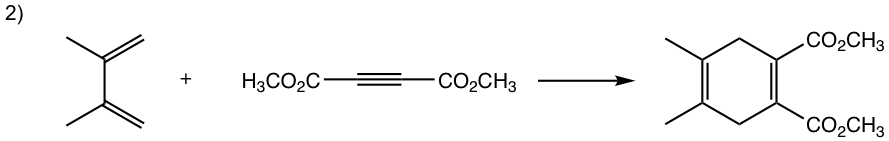
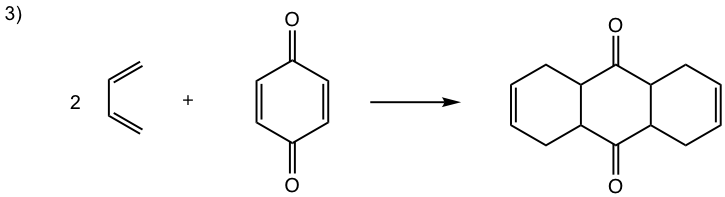
Show the product of the following Diels-Alder reactions.
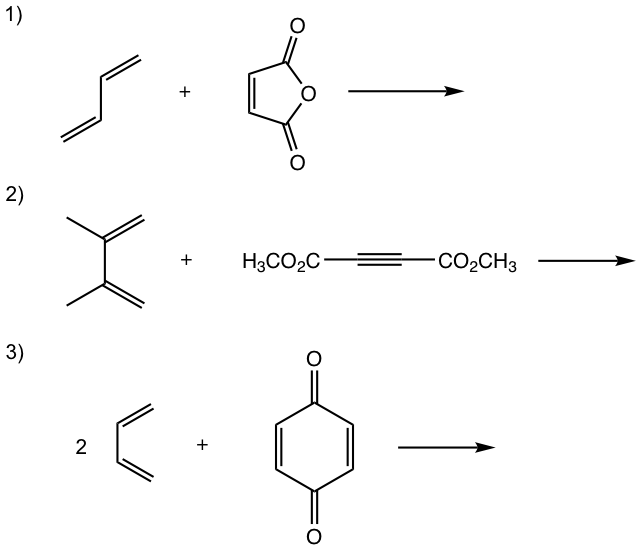
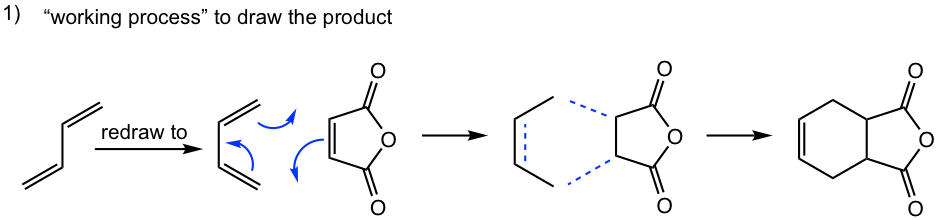
Tips to work out the D-A reaction product: (reaction #1 is shown as example for the process)
- If the diene is given in s-trans conformation, redraw it in s-cis conformation.
- Arrange the diene in a way that the carbon atoms at the ends of both double bonds are close to the double bond in the dienophile.
- Connect the atoms, and redraw the bonds: two new σ bonds and one new π bond in the product ring.
Answers:
3.5.3 Stereochemistry of the Diels-Alder Reaction
Syn addition
As a concerted cyclization process, the Diels-Alder reaction is a syn addition, which means one face of the diene adds to one face of the dienophile. As a result, the configurations of the diene and dienophile are retained in the product (Fig. 3.5i).
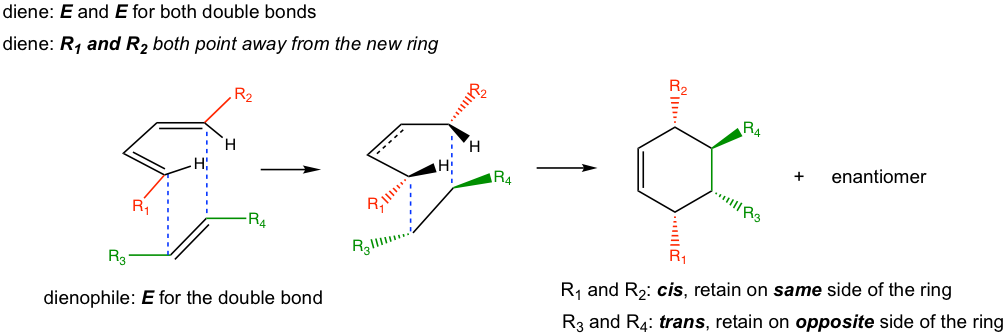
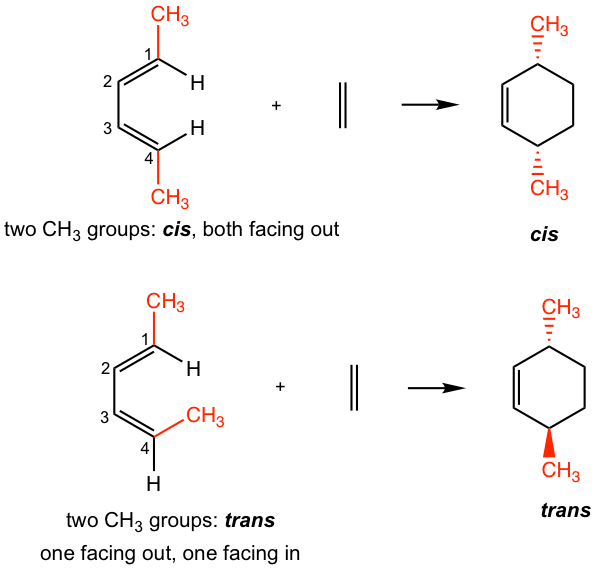
— For dienes: For dienes that have substituents on C-1 and C-4, the new stereocenters will be formed in the cyclohexene product. The two substituents of diene can be regarded as being either “cis” (both facing in or both facing out) or “trans” (one facing in and one facing out). The stereochemistry of these substituents in the cyclohexane product is retained as “cis” or “trans” respectively, as shown in Fig. 3.5j.
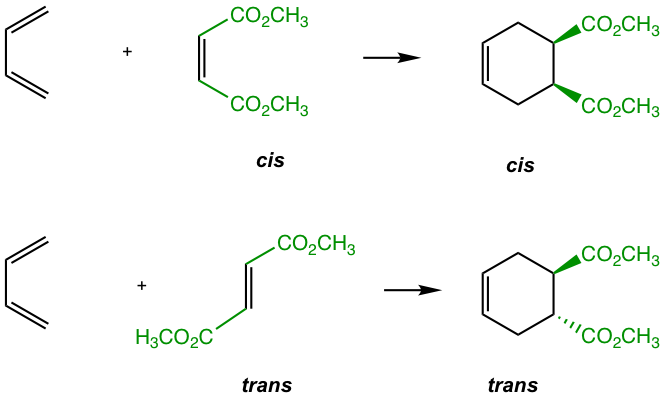
— For dienophiles: The cis substituents of the dienophile will retain cis in the substituted cyclohexane product, and the trans substituents of the dienophile will retain trans in the substituted cyclohexane product.
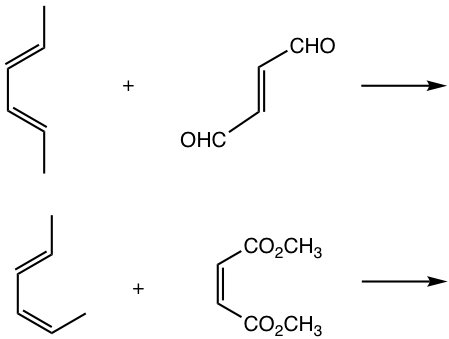
Examples
Show products of the following reactions.
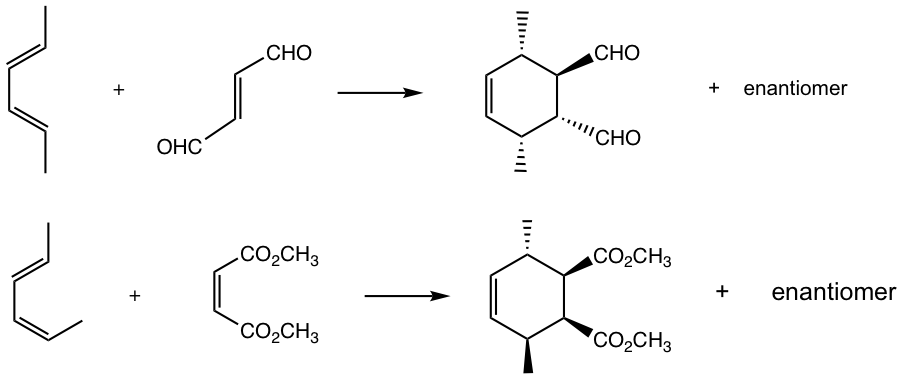
Answers:
Endo Rule
Another stereochemistry characteristic of the Diels-Alder reaction is that only the endo product is formed. The terms endo and exo are used to indicate the orientation of substituents in a bicyclic ring system. The endo position on a bicyclic structure refers to the position that is closer (or inside) to the concave shape of the larger (six-membered) ring, and the exo position is away from (or outside) the larger ring as shown in Fig 3.5l.
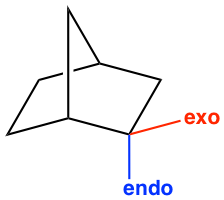
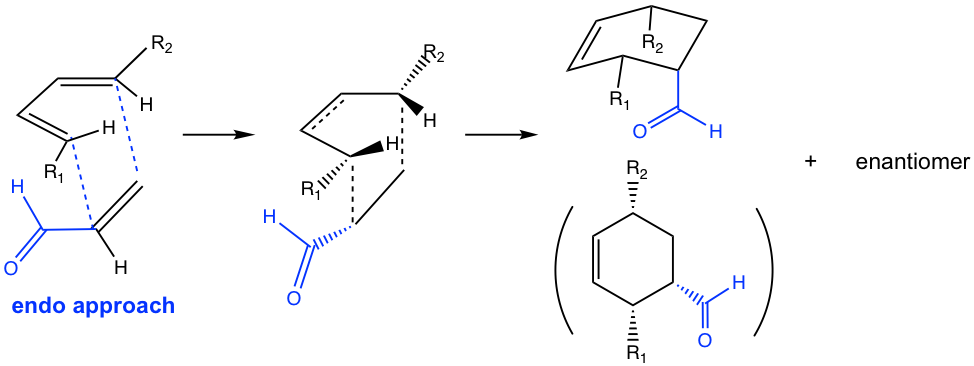
In the Diels-Alder reaction, endo or exo refers to the orientation of the dienophile when it reacts with diene. A good dienophile, as mentioned earlier, always has an e-withdrawing group, such as a carbonyl-containing group. When the π electron orbitals in the carbonyl group align under (or above) the π electron orbitals on the diene, the orientation of the approach is called endo, as shown in Fig. 3.5m. The endo approach allows the orbital overlapping of the carbonyl group with the diene, and lowers the energy of the transition state, therefore is the favorable way of the reaction.
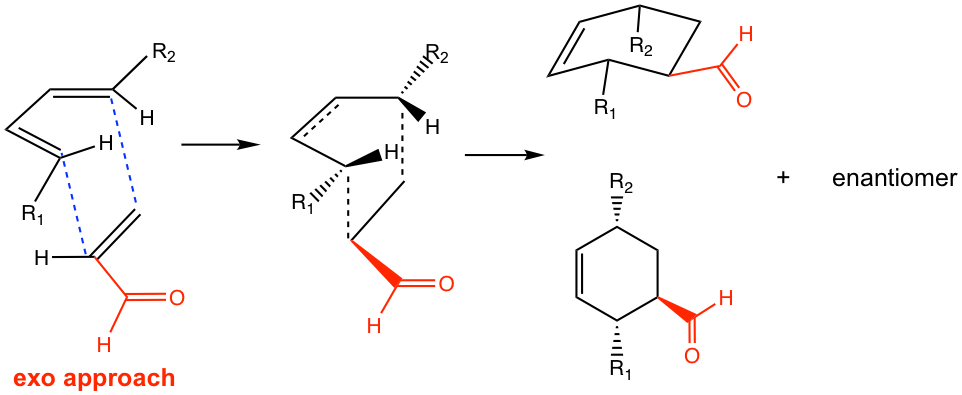
If the reaction proceeds in the exo approach, on the other hand, the π electron orbitals in the carbonyl group align away from the π electron orbitals on the diene.
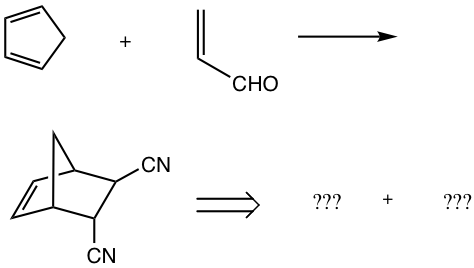
Examples
Show the product or the reactants of the following reactions.
Answers: