Chapter 3: Conjugated Unsaturated Systems
3.2 Reactions of Allyl System
Radical Halogenation (Substitution) of Allylic Substrates
As mentioned earlier allylic hydrogens are especially reactive in radical halogenation (substitution) reactions. The reaction proceeds through the same mechanism as alkane halogenation that we learned before (refer to section 9.2 in Book I). When halogens, Cl2 or Br2, are employed for such reactions, however, addition reactions to the double bond readily occur as well. To avoid the competition of addition and promote substitution, high temperature and low concentration of halogens should be maintained to give the allylic halogenation product.

As an alternative to halogen reagents, N-bromosuccinimide (NBS) is the most common reagent applied for allylic bromination.
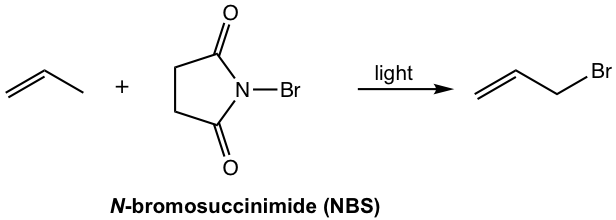
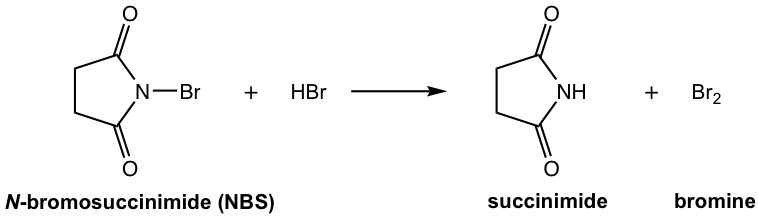
N-bromosuccinimide (NBS) is a solid reagent. By reacting with a trace amount of HBr (usually present in the solvent, such as CCl4), NBS can provide a constant but low concentration of bromine, Br2. A low concentration of Br2 facilitates substitution (allylic bromination), and avoids the competition of addition.
Examples
Show the monobromination product of the following reaction.
![]()
Answers: A mixture of two constitutional isomers obtained as products.
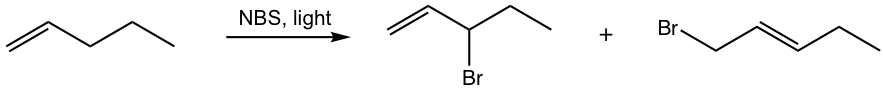
Analysis: The mechanism explains why. There are two non-equivalent resonance structures for the allyl radical intermediates, each leads to a bromination product.

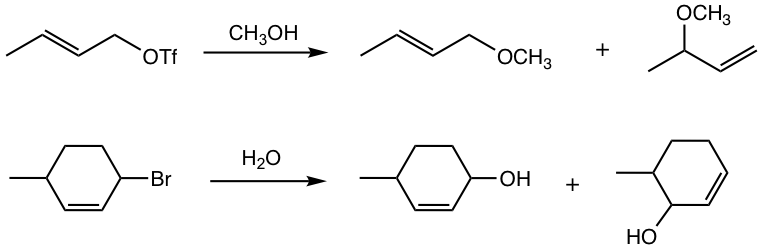
Nucleophilic Substitution of Allylic Substrates
As the example shown in Fig. 3a, the substrate that forms an allyl carbocation undergoes the SN1 reaction readily. A few more examples are given in Fig. 3.2c. Multiple products were obtained because of the non-equivalent resonance structures for the allyl carbocation intermediate.