Chapter 2: Aldehydes and Ketones
2.5 Addition of Alcohols to Aldehydes and Ketones
The carbonyl group in aldehyde and ketone also reacts with alcohol, another nucleophile, in the presence of an acid catalyst. The initial nucleophilic addition gives hemiacetal as a product, and the reaction continues with another equivalent of alcohol to form an acetal.
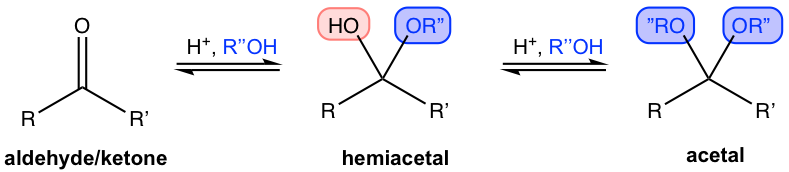
Formation of Hemiacetals
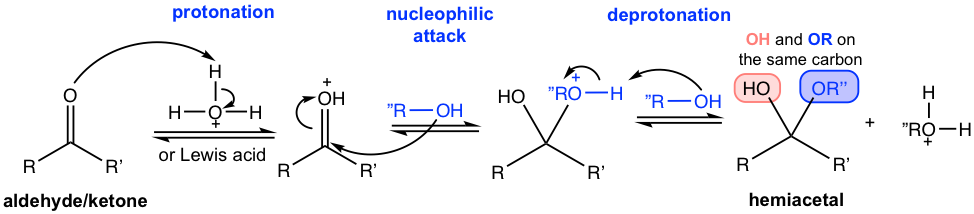
The mechanism for hemiacetal formation fits in the general Mechanism II (Fig. 2.3d) pretty well, which involves protonation, nucleophilic attack by alcohol, and deprotonation.
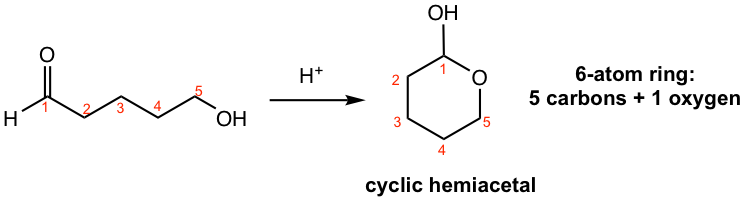
Hemiacetals are usually not stable enough to be isolated as the product. The special case is cyclic hemiacetals with 5- or 6-membered rings that can be isolated because of the stability of the ring.
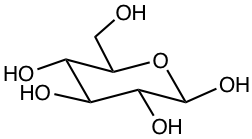
One typical example of cyclic hemiacetal is sugar. For example, the majority of glucose molecules adopt the cyclic hemiacetal structure in a solution of glucose (Chapter 9).
Formation of Acetals
The mechanism from hemiacetal to the formation of acetal is shown in Fig. 2.5e.
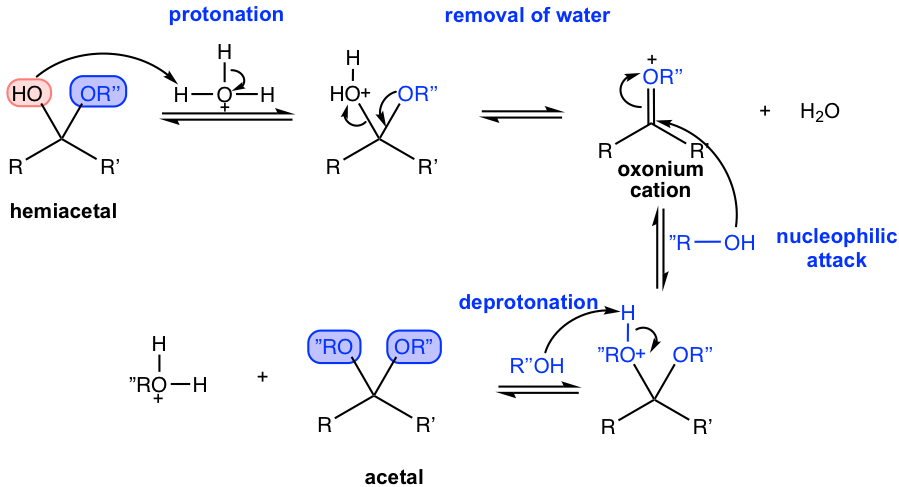
Starting from hemiacetal, protonation occurs again on the OH group. The reason for this protonation is to transfer OH (in hemiacetal) to H2O, or a poor leaving group to a good leaving group, so water could be removed. After the removal of water, another alcohol does the nucleophilic attack on the oxonium cation intermediate, which leads to the product acetal with another deprotonation.
Since all the steps for the formation of hemiacetals and acetals are reversible, several practical tips are usually applied in the reaction to achieve a high yield of the product. First, a large access of alcohol is usually added to the reaction because of the availability of alcohol. Secondly, water, the side product of the reaction, is removed from the reaction process. Both measures help the equilibrium to strongly favor the product side and ensure a good yield of the acetal.
Notes for the mechanism of hemiacetal and acetal formation:
The mechanism for the formation of hemiacetal and acetal involves multiple steps, it might feel overwhelmed when you learn that the first time. They are very important mechanisms though, and being able to draw the full mechanism (including the mechanism for the reverse process, i.e., hydrolysis of acetal, in the following section) is part of the learning objective for the course at this level. There are a few tips to help you understand and memorize the mechanism more easily.
- Nucleophilic addition is the keyword, and alcohol (ROH) works as the nucleophile to attack the carbonyl carbon in these mechanisms.
- Since alcohol is a weaker nucleophile, protonation occurs first in the presence of acid and the protonated carbonyl is more reactive with alcohol nucleophile.
- Another protonation step is involved from hemiacetal to acetal, but for different purposes. In this part of the mechanism, protonation helps to transfer OH (the poor leaving group in hemiacetal) to a good leaving group H2O, and then water could be removed.
- The mechanism is always product-oriented. In which way it makes sense to achieve the product, the reaction proceeds that way likely.
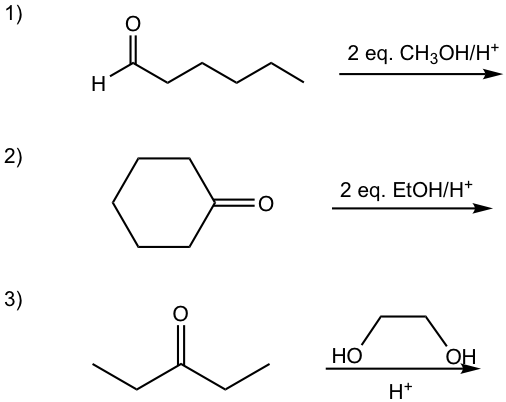
Examples:
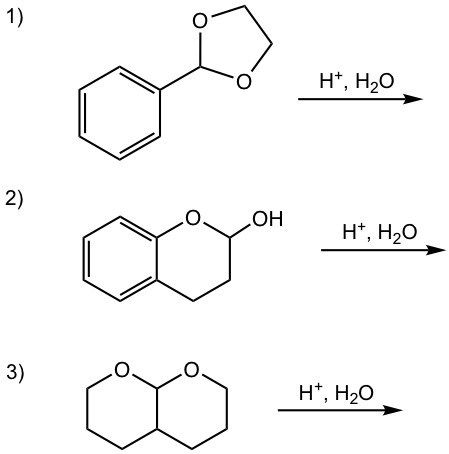
Show the product of the following reactions.
Answers:
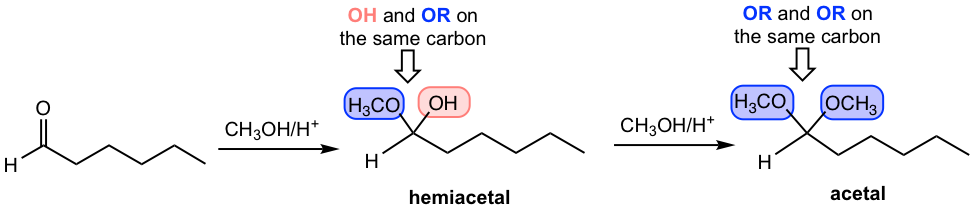
1)
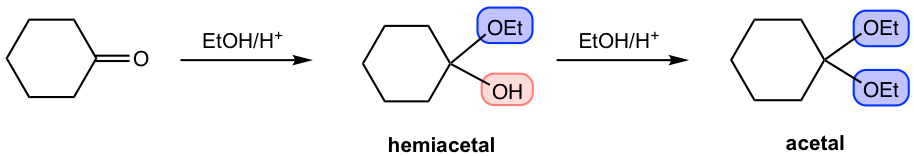
2)
3)
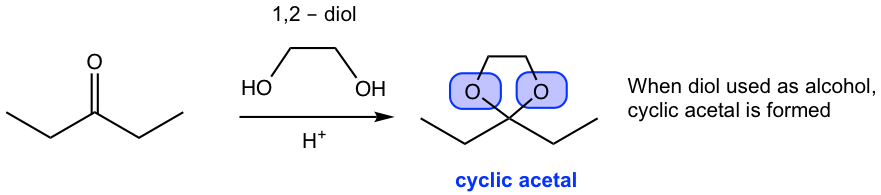 As shown in the last example #3), cyclic acetal is formed when diol is used as alcohol. Other diols that could be used for cyclic acetals include 1,3-propanediol, 2,2-dimethyl-1,3-propanediol.
As shown in the last example #3), cyclic acetal is formed when diol is used as alcohol. Other diols that could be used for cyclic acetals include 1,3-propanediol, 2,2-dimethyl-1,3-propanediol.

Notes regarding the structures of hemiacetals and acetals:
Being able to recognize the structures of hemiacetals and acetals is critical. Both functional groups are brand new to us, and both have unique structure features. The key structure of hemiacetal is that one OH and one OR are both attached to the same carbon atom, which is kind of a “mixed structure of alcohol and ether”. For acetal, on the other hand, the key feature is that two OR groups share the same carbon. As shown in the above examples, the groups are highlighted in different colors to make it easier for you to recognize and compare. It is more challenging to recognize when the ring is involved in the functional group, see practice question #1 at the end of the chapter.
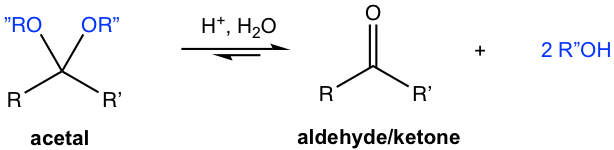
Hydrolysis of Acetal
In the preparation of acetal, as it was mentioned earlier, water should be removed from the reaction system to favor the equilibrium of the product side. On the other side, if acetal is placed in excess water with acid present, acetal undergoes hydrolysis and goes back to aldehyde or ketone.
Examples
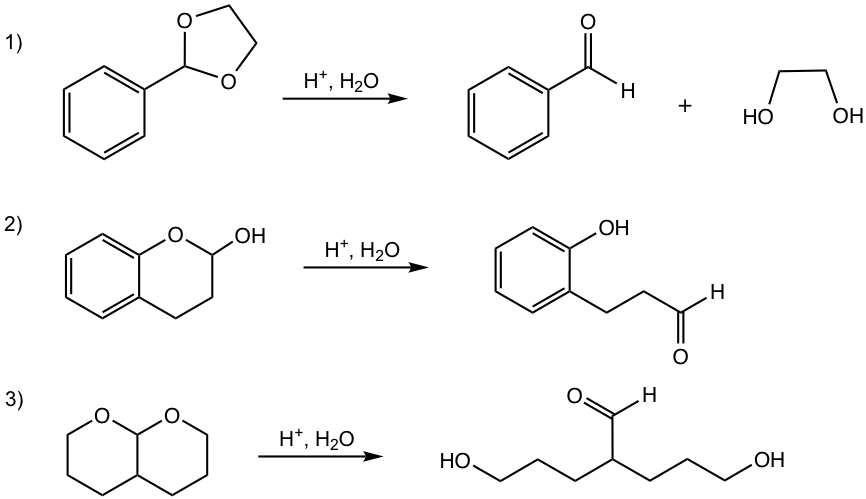
Examples
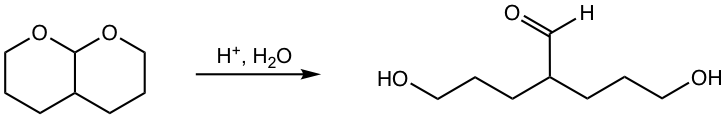
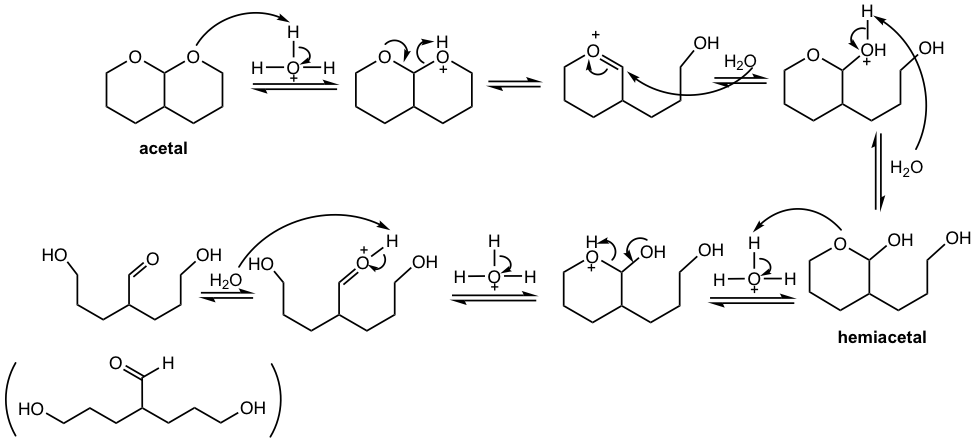
While acetals undergo hydrolysis in an acidic aqueous solution, they are stable and barely undergo any reactions in basic conditions.

