Chapter 2: Aldehydes and Ketones
2.4 Addition of Water to Aldehydes and Ketones
Aldehydes undergo the addition of water under either acidic or basic conditions. The addition of water can also be called hydration, that produces hydrate of aldehyde or ketone.
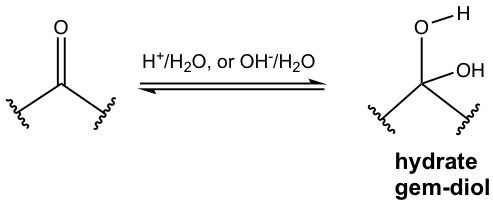
In the hydration product, a water molecule (H-OH) is added to the C=O bond, specifically the OH group is connected to the carbon atom, and the H bonds to the oxygen atom. The product, therefore, has two OH groups on two adjacent carbons, such functional group is also called the geminal diol (gem-diol).
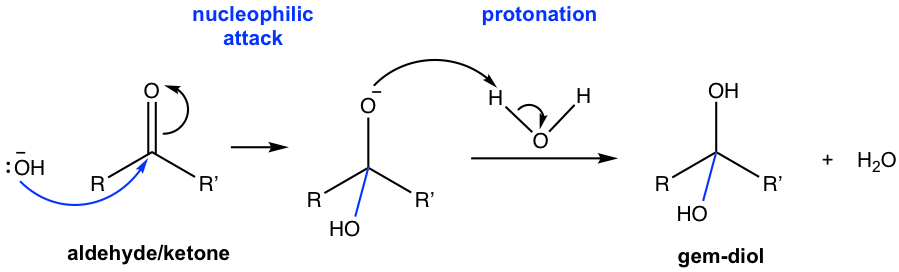
The mechanism for hydration reaction in basic conditions fits in mechanism I (Fig. 2.3c), and that in acidic conditions is a good example of mechanism II (Fig. 2.3d). The detailed mechanisms for hydration are shown below.

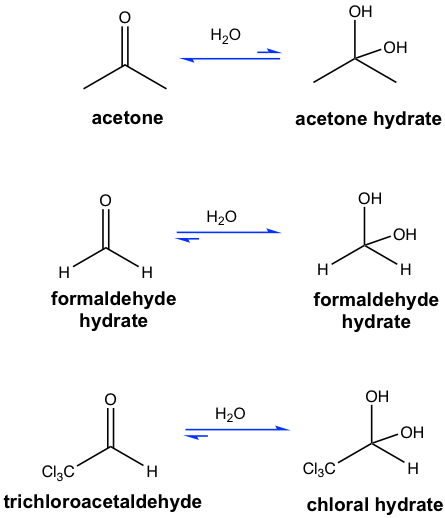
In most cases, the resulting hydrate (gem-diol) is unstable and cannot be isolated. The effort of heating to isolate hydrate results in evaporation of water, and pushes the equilibrium to the carbonyl compound side, and the hydrate is converted back to carbonyl compound.
Because of the relatively low reactivity of ketone towards nucleophiles, the equilibrium for the hydrate of ketone is unfavorable. On the other side, the hydrate predominates the equilibrium for some aldehydes (Fig. 2.4d). One example is that the aqueous solution of formaldehyde (formalin) contains primarily the hydrate CH2(OH)2. Also, the presence of strong electron-withdrawing groups destabilizes the carbonyl group and tends to form stable gem-diols. The equilibrium of chloral hydrate provides a typical example of such a case (in Fig. 2.4d).