Chapter 1: Alcohols and Ethers
1.7 Practices Answers
- Show the product of the following reactions.
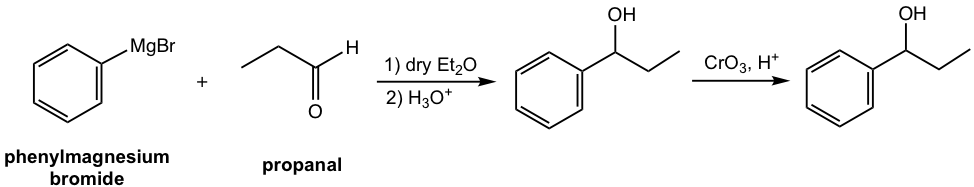
a.
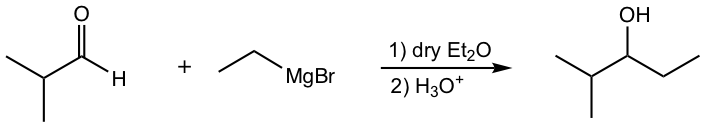
b.
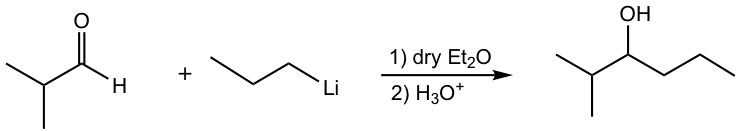
c.
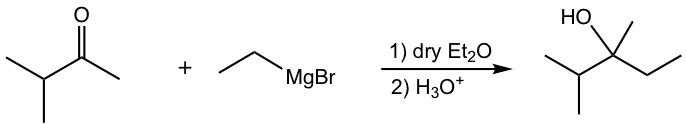
d.
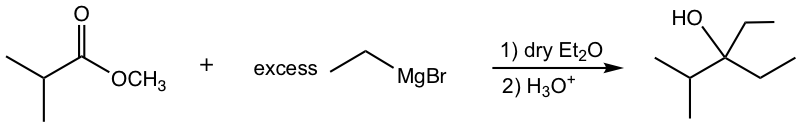
e.
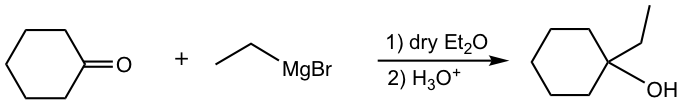
f.
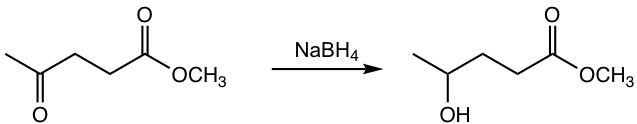
g.
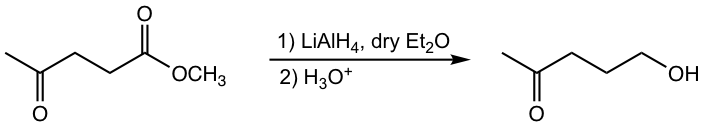
h.
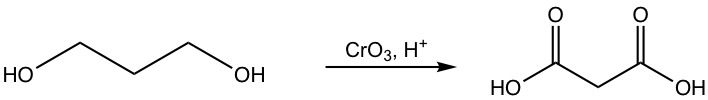
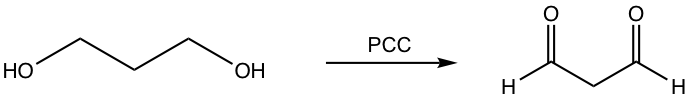
i.
2. Show the detailed steps to synthesize the following ethers using Williamson synthesis. Other reagents and solvents can be used as necessary.
Tips for 1): To keep the configuration of the reactant retained in the product, the chirality center should not be involved in the reaction, therefore the reactant S-2-pentanol should be converted to the nucleophile, and reacts with halide. The bond-breaking position is shown below.
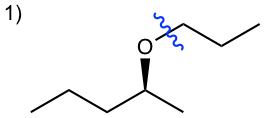
Answer for 1):
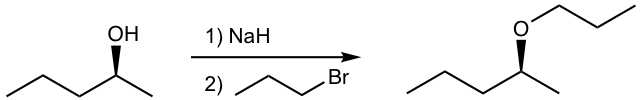
Tips for 2), to get the inversed configuration in the product, the SN2 reaction should occur on the chirality center, therefore the S-2-pentanol should be converted to a halide (LG), and react with another nucleophile. The bond-breaking position is shown below.

Answer for 2):
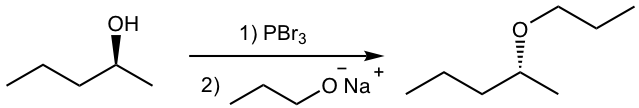
3. Propose the synthesis for each of the following compounds, based on the starting materials specified.
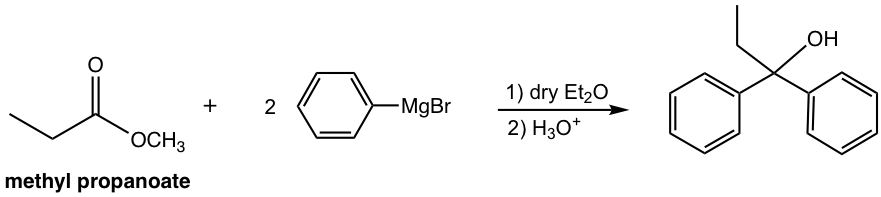
a. Start with methyl propanoate and other necessary chemicals/reagents.
Retrosynthesis analysis:
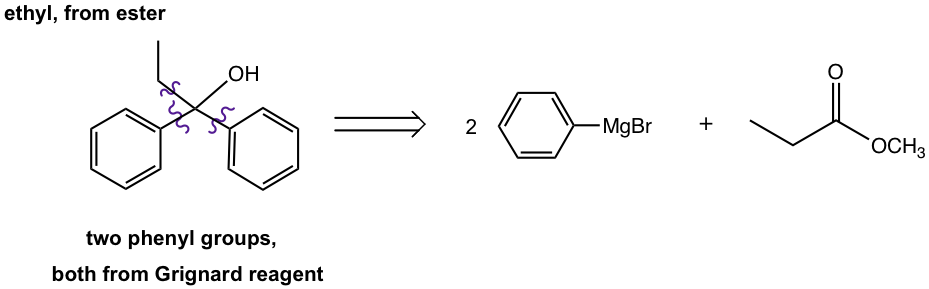
Answer:
b. Start with phenylmagnesium bromide and other necessary chemicals/reagents.
Retrosynthesis analysis:
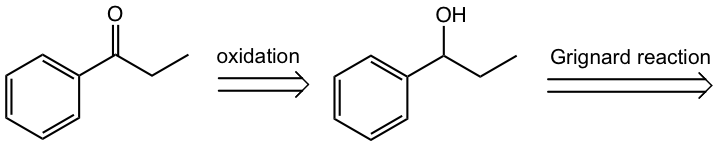
Answer: