Chapter 6: Reaction at the α-Carbon of Carbonyl Compounds
6.5 Reactions of α,β-unsaturated Aldehydes and Ketones
α,β-unsaturated aldehydes and ketones are the products of aldol condensations. Two functional groups, C=C bond alkene, and C=O bond carbonyl, presented in this type of compound in the conjugated way. The reactivities of α,β-unsaturated aldehydes and ketones therefore are determined by such special structure features.
As we have learned the C=O bond is ready to undergo nucleophilic addition (section 2.3). Similarly, the addition is also the most typical reaction for the C=O bond in α,β-unsaturated aldehydes and ketones. However, depending on the structure of the nucleophile, the nucleophilic reaction could take place in two different ways: simple addition in which the addition just occurs in the C=O group, or conjugate addition in which the C=C bond is involved in the addition as well.
Simple addition is also called 1,2-addition, and the conjugate addition is usually named 1,4-addition, as illustrated below in Fig. 6.5a. The numbers here (1,2 or 1,4) are for the purpose of labeling the atoms involved in the conjugated system, and have nothing to do with the numbering in the IUPAC name of the compound. The structures shown in Fig. 6.5a are general structures.
Why the addition could be either 1,2- or 1,4-? We can get the answer by examining the resonance structures of α,β-unsaturated carbonyl compounds (Fig. 6.5b).
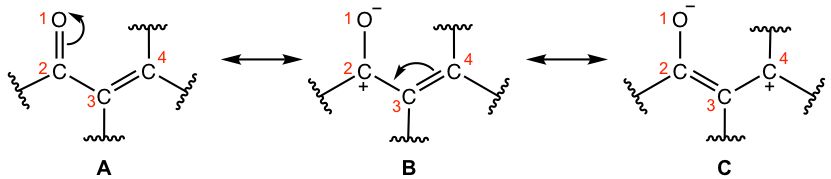
Both resonance structures B and C make significant contributions to the hybrid since the negative charge is on oxygen, the atom with high electronegativity. It is indicated in structures B and C that both carbonyl carbon (C-2) and the β-carbon (C-4) bear a partial positive charge, so they both are spots where the nucleophilic attack could occur, therefore the reaction could be either 1,2- or 1,4-addition.
6.5.1 1,2-addition of α,β-unsaturated aldehydes and ketones
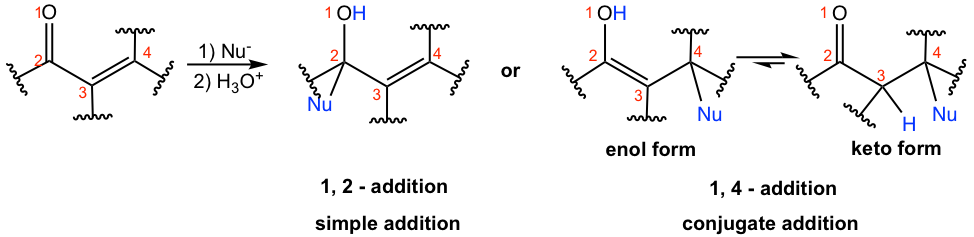
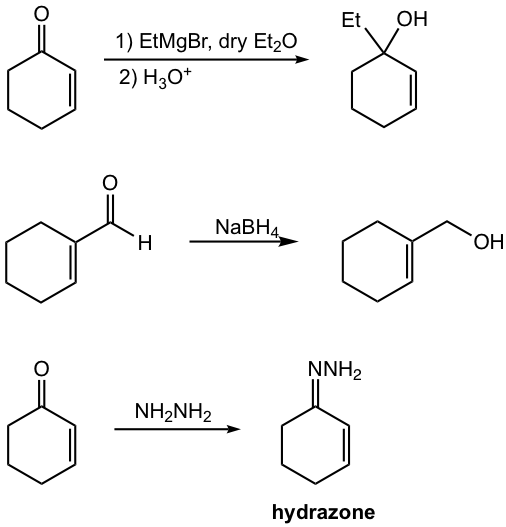
1,2-addition is favored when strong nucleophiles are applied to the reaction. Strong nucleophiles include the nucleophile with negative charge, for example: hydride (H–), lithium reagent (RLi), and Grignard reagent (RMgX). The reaction mechanism is the same as simple nucleophilic addition to the carbonyl group that we learned in section 1.4. Another type of reagent that gives a 1,2-addition product is the derivatives of primary amine, which includes hydroxylamine NH2OH, hydrazine NH2NH2, and semicarbazide NH2NHCONH2. The 1,2-addition products of these compounds are precipitates that serve as the driving force to make this pathway favorable (refer to section 2.7)
6.5.2 1,4-addition of α,β-unsaturated aldehydes and ketones
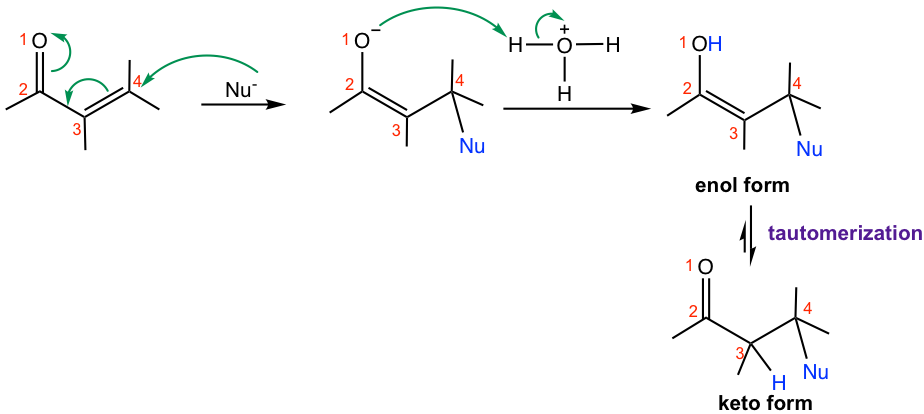
Generally speaking, when weaker nucleophiles are used, the 1,4-addition product is formed as the major one. The weaker nucleophiles could be H2O, ROH, amines, and CN– followed by protonation. The 1,4-addition example of methyl amine to 2-cyclohexen-1-one is shown in Fig. 6.5d.
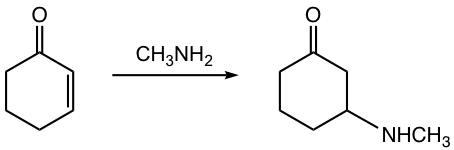
The mechanism for 1,4-addition of α,β-unsaturated compounds is shown in Fig. 6.5d. Nucleophile attacks at the β-carbon (C-4) first, and the enolate intermediate accepts a proton to give the enol form product. Enol form compound undergoes tautomerization automatically because of the higher stability of the keto form and produces keto form as the final product. Please note that the final product seems to arise from 3,4-addition, but it is actually through the 1,4-addition mechanism!
6.5.3 Organocuprate Addition
Here we will introduce a type of new organometallic reagent, organocuprate, that allows 1,4-addition to α,β-unsaturated aldehydes and ketones. An example is given here that the ethyl group is added to the β-carbon of 2-cyclohexen-1-one.
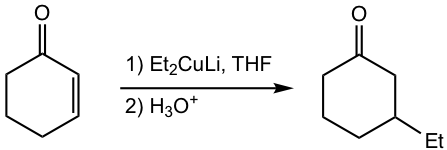
The specific organocuprate reagent applied here is lithium dialkylcopper (R2CuLi). It is the source of nucleophiles similar to Grignard and organolithium reagents, however, with slightly weaker reactivity, and adds to α,β-unsaturated aldehydes and ketones in the 1,4-addition pathway.
Organocuprate reagents are made from the reaction of organolithium reagents and copper (I) iodide, CuI.

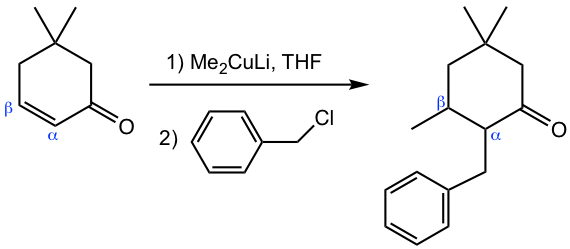
While organocuprate provides a way to introduce an alkyl group into the β-carbon, applying organocuprate followed by the alkylation step could introduce two alkyl groups into the β-and α-carbons separately, as shown in Fig. 6.5h.
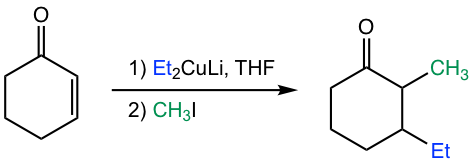
The understanding of this reaction comes from the reaction mechanism (Fig. 6.5i).
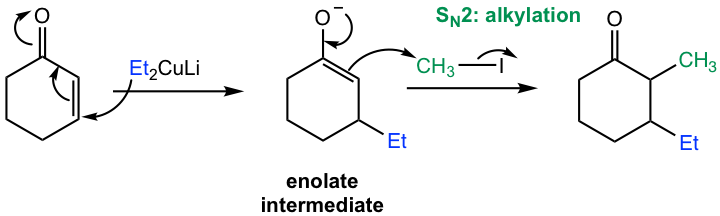
In the first nucleophilic addition step with organocuprate, the enolate intermediate is formed. Instead of protonation as in the common 1,4-addition process, here the enolate reacts with an alkyl halide in an SN2 reaction in the 2nd step, as we learned in section 6.3 that enolate is available for alkylation. Overall, the organocuprate reagent followed by alkylation with alkyl halide is a method to achieve α,β-dialkylation (the two alkyl groups could be either the same or different) to α,β-unsaturated aldehydes and ketones, and therefore has great applications in synthesis.
Examples
Show the product of the following reaction.
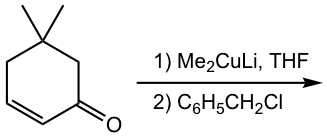
Answer:
6.5.4 Michael Addition
Michael addition is the 1,4-conjugate addition of enolate ion to α,β-unsaturated carbonyl compound. Enolate ion acts as the nucleophile in Michael addition.
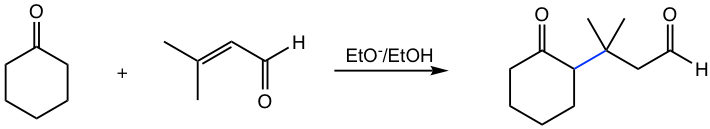
The mechanism for Michael addition follows the general way of 1,4-addition, with enolate acting as the nucleophile, as shown in Fig. 6.5k. Enolate attacks the β-carbon (C-4) first, and then the protonation with the solvent of the enolate intermediate gives the final product. Alkoxide, RO–, is the base commonly used for Michael addition to generate enolate, together with the corresponding alcohol ROH as solvent.
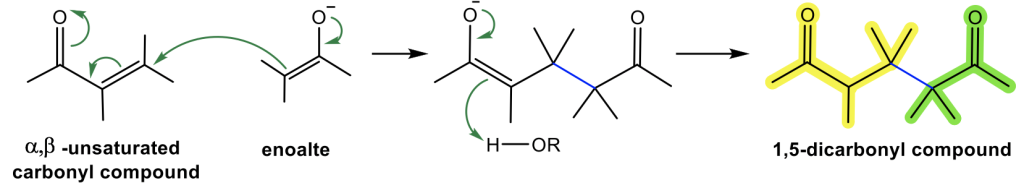
Michael addition is an effective way to build larger molecules by connecting two structure units with a new C-C bond (blue bond in Fig. 6.5k). When Michael addition takes place between the enolate of an aldehyde or ketone with α,β-unsaturated aldehyde or ketone, the 1,5-dicarbonyl compound is formed as a product.
Michael addition takes place with a variety of other reagents as well, as the examples shown in the following table, demonstrate the diverse applications of Michael addition in synthesis.
| Compound for enolate | α,β-unsaturated compound | Michael addition product |
|
1)
|
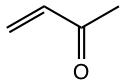 |
 |
| 2)
|
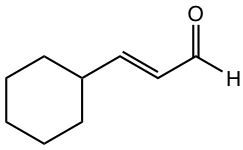 |
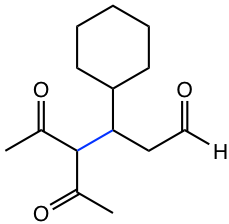 |
| 3)
|
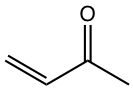 |
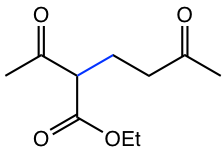 |
Table 6.1 Examples of Michael addition with a variety of compounds that generate enolates
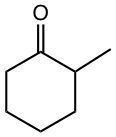
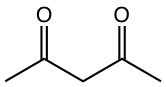
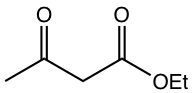
As you may have noticed in the last two examples in Table 6.1, the compounds to generate enolate are not the usual aldehyde or ketone. These two examples represent a type of compound called β-dicarbonyl compound. The uniqueness of β-dicarbonyl compound is that the hydrogens on the carbon between the two carbonyl groups are even more acidic than α-hydrogens in the carbonyl compound with one C=O group. The pKa of several types of β-dicarbonyl compounds, in the range of 9 ~ 13, are shown in Fig. 6.5l.
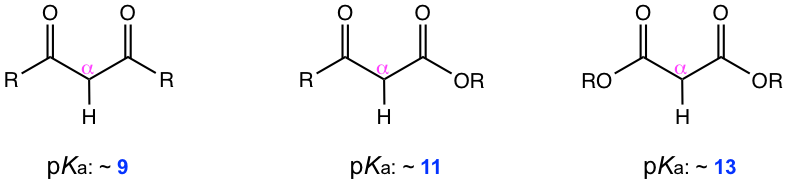
The reason for the greater acidity of α-hydrogens in β-dicarbonyl compounds is apparently due to the presence of another C=O bond. The delocalization of negative charge onto two oxygen atoms instead of one helps to stabilize the enolate significantly (Fig. 6.5m), therefore making the hydrogens more acidic.

The stabilized enolates of β-dicarbonyl compounds are used broadly in organic synthesis, and we will see more applications in Chapter 8. Two specific compounds are particularly important and need our attention, they are acetoacetic ester and diethyl malonate.

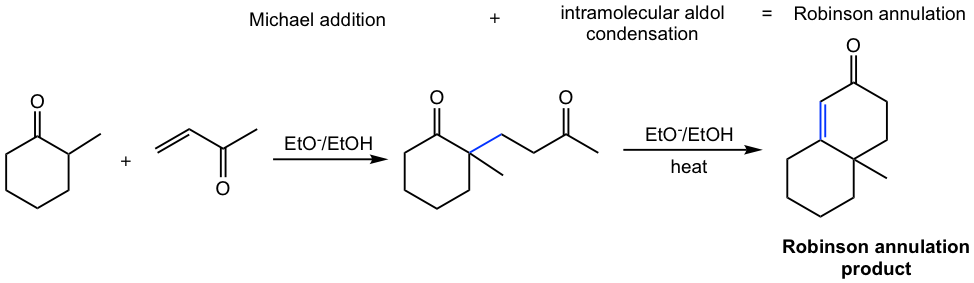
6.5.5 Robinson Annulation
The Michael addition followed by an intramolecular aldol condensation is called Robinson annulation. The term annulation means ring-forming because a ring is formed by intramolecular aldol condensation. The product of a 5- or 6-membered ring is usually formed in Robinson annulation because of the stability of the ring.
For the first example in Table 6.1, the product undergoes further intramolecular aldol reaction and gives a bicyclic product, and the overall reaction is a Robinson annulation.