Chapter 6: Reaction at the α-Carbon of Carbonyl Compounds
6.3 Alkylation at the α-Carbon
In this section, we will have further discussions about how to generate enolate anion and the reactions of enolate.
Preparation of Enolate Ion
In earlier sections, we did see that a common base like OH– could remove α-hydrogen. However, the amount of enolate ion generated by using OH– is rather small, because OH– is a weaker base than the enolate and the reaction equilibrium mainly lies on the reactant side (refer to acid-base reaction equilibrium in section 3.3 in Book I).
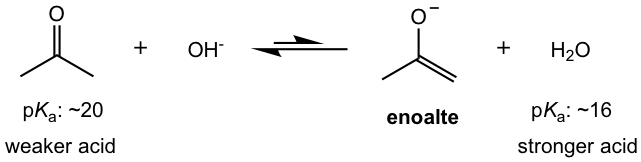
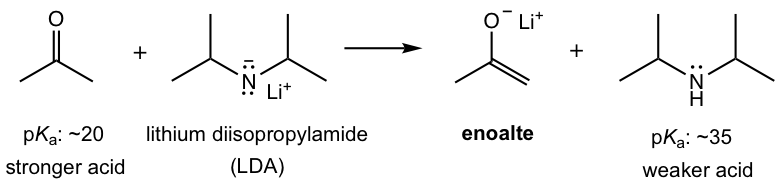
To produce enolate in good yield, a stronger base is therefore required. The most common base employed for this purpose is lithium diisopropylamide (LDA). As the conjugate base of a much weaker acid diisopropylamine (with pKa of about 35), LDA is a much stronger base than enolate and the equilibrium lies on the product (enolate) side predominantly. LDA is the choice of base for the reactions that require carbonyl compound to be completely converted to enolates before further reactions.
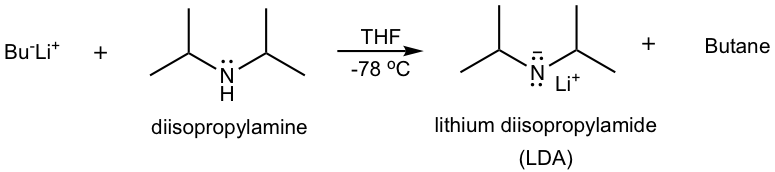
LDA is a strong bulky base that can be prepared by adding alkyllithium to diisopropylamine in a polar aprotic solvent such as THF at the low temperature of -78 °C. Low temperature is usually required to prepare and handle LDA because of the high reactivity.
For asymmetric ketone that has α–hydrogens on different α–carbons, such as 2-methylcyclohexanone, the enolate could be generated on either side depending on the reaction condition employed (Fig. 6.3d).
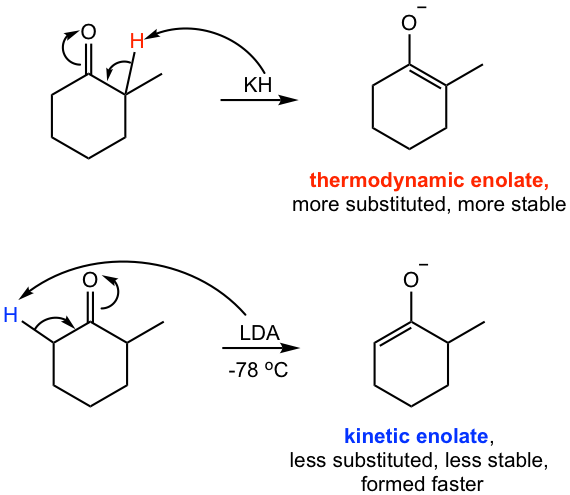
The enolate ion formed by the removal of the α-hydrogen (red one) on the more sterically hindered side is highly substituted and, therefore is thermodynamically more stable, so is called the thermodynamic enolate. The other one formed by the removal of the α–hydrogen (blue one) on the less sterically hindered side is less substituted, not as stable, but is formed faster because of less steric hindrance, such enolate is called the kinetic enolate. The use of a strong bulky base, such as LDA, at low temperature favors the formation of a kinetic enolate, and the use of a small size base, such as KH, at higher temperature favors the formation of a thermodynamic enolate.
Direct Alkylation via Enolate
The enolate ion, with a negative charge, is nucleophilic and reacts with electrophiles. When enolate reacts with an alkyl halide, the reaction proceeds in a similar way as the SN2 reaction, and an alkyl substituent is introduced onto the α carbon. In the following example, the ethyl group is introduced onto a carbon of cyclohexanone by a two-step process.

This is an important and useful reaction in organic synthesis because it provides another way to form carbon-carbon bond, and build larger molecules. Because it is an SN2-type reaction, it works best with methyl and primary alkyl halides.
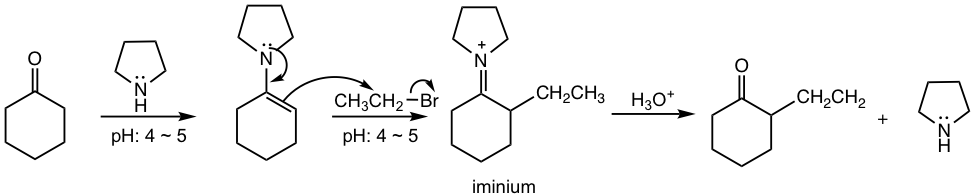
Indirect Alkylation via Enamine
An alternative way of alkylation is via the enamine derivative of aldehyde and ketone. Recall that enamines are produced when aldehydes or ketones react with secondary amine (section 2.7.3), and enamine is also a good nucleophile and can react with alkyl halides in SN2-type reactions to produce iminium ion. The hydrolysis of the iminium ion intermediate gives the alkylation product. This method offers another way to add an alkyl group to the α-carbon of a carbonyl compound.