Chapter 5: More Reactions on Aromatic Compounds
5.5 Arenediazonium Salt
Preparation and Structure of Arenediazonium Salt
Aniline can be converted to an arenediazonium salt by reaction with nitrous acid (HNO2). The arenediazonium salt can undergo further substitution and coupling reactions which are important methods to produce more benzene derivatives.
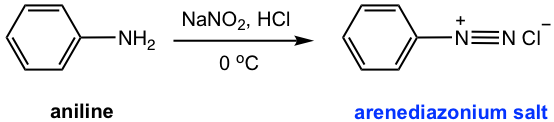
When treated with nitrous acid (HNO2) at 0 °C, the NH2 group on aniline is converted to the arenediazonium salt.
Nitrous acid is an unstable acid that has to be prepared in situ, by treatment of sodium nitrate (NaNO2) with strong acid solution (HCl), and the reaction system needs to be kept at 0 °C. The diazonium ion then generated can be readily replaced by a variety of nucleophiles (as shown in Fig. 5.5b and Fig. 5.5d), with the formation of nitrogen gas, a stable molecule, which acts as a driving force to facilitate the reaction. The nucleophile reagent can be simply added to the mixture, with no separation of diazonium salt necessary.
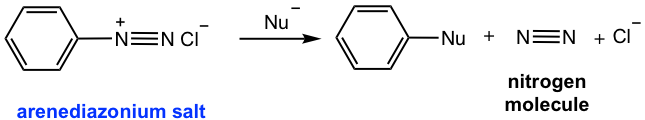
As a reactive intermediate, arenediazonium ion is relatively more stable than alkanediazonium because of the resonance effect from the benzene ring. As shown in Fig. 5.5c, multiple resonance structures are available for arenediazonium ion.
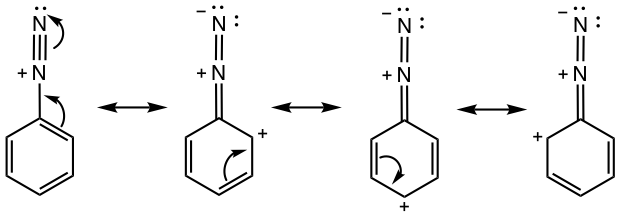
Syntheses of Substituted Benzene by Using Arenediazonium Salt
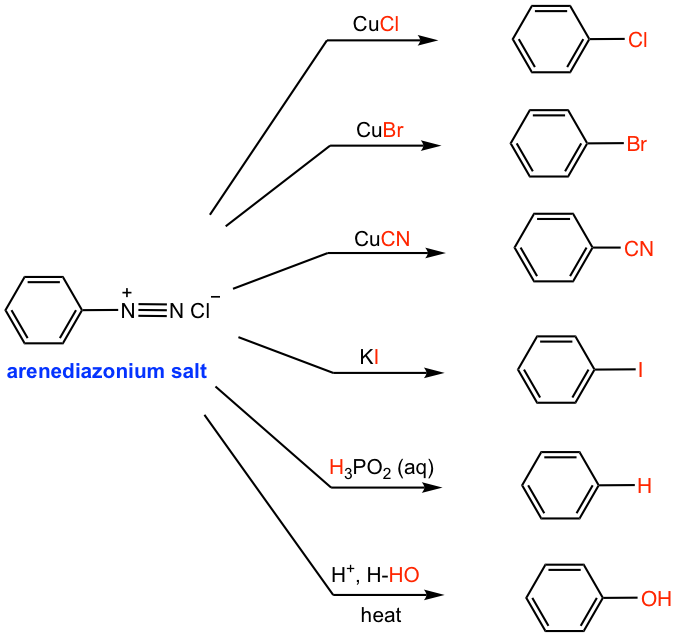
Arenediazonium salt undergoes a series of substitution reactions, with a variety of substituted benzene generated as products (Fig. 5.5d). The reactions of several copper (I) salts, for example, CuCl, CuBr, and CuCN, with arenediazonium salt are generally known as Sandmeyer reactions. The diazonium group is replaced by -Cl, -Br, and -CN, respectively, in these reactions.
To prepare the iodobenzene from arenediazonium salt, potassium iodide (KI) is required.
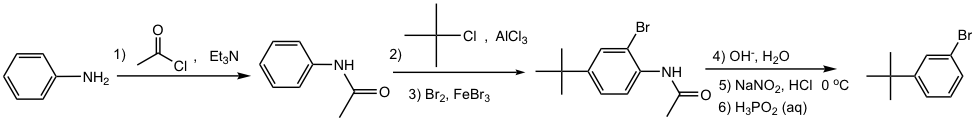
A useful reaction in synthesis is the replacement of diazonium salt with a hydrogen atom, which is also called deamination (removal of the nitrogen group). This can be achieved by treatment of arenediazonium salt solution with hypophosphorous acid (H3PO2) aqueous solution (the 2nd last reaction in Fig. 5.5d). This reaction is useful if the -NH2 group (or the -NO2 group, the precursor of -NH2) is needed for directing purposes, and must be removed afterward. Such an application is illustrated in the following synthesis example.
Examples
Design the synthesis of m-bromo-t-butylbenzene from aniline.
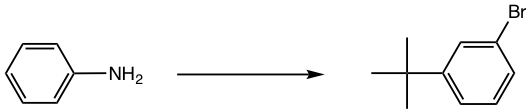
Approach:
- NH2 is first converted to NHCOR, a milder activator and also ortho-para director;
- t-Butyl group is then introduced by Friedel-Crafts alkylation, the para isomer will be obtained as a major product because of steric effect;
- Br is introduced next by bromination;
- NHCOR is hydrolyzed back to the NH2 group, which is converted to diazonium salt, and then replaced by H.
Answer:
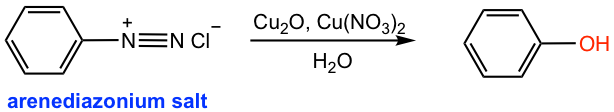
If the acidic solution containing arenediazonium salt is warmed up, the -OH group will replace the diazonium salt in which H2O acts as the nucleophile (the last reaction in Fig. 5.4d). This is a method to prepare phenol from aniline.
Another better alternative to preparing phenol (Fig. 5.5e) is to add copper(I) oxide and copper(II) nitrate to the cold solution of arenediazonium salt. The diazonium salt is replaced by the -OH group in such conditions without heating.
Coupling Reaction of Arenediazonium Salt
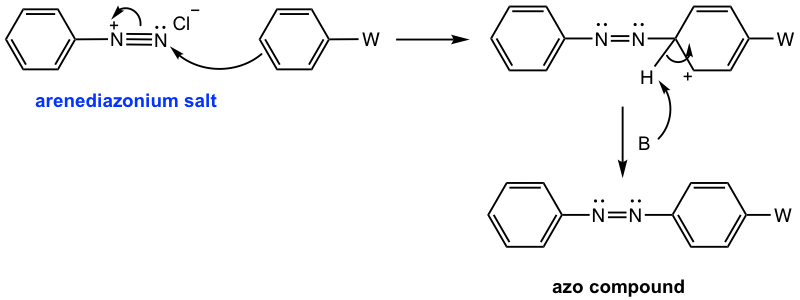
The arenediazonium salt can also react as an electrophile in the electrophilic aromatic substitution (EAS) reaction. Since arenediazonium salts are weak electrophiles, highly activated aromatic compounds, such as phenol or aniline, are required. The product of such a reaction contains an N=N linkage structure unit. The N=N is called an azo linkage, and the product is called the azo compound. The azo compound comes from the connection of arenediazonium salt with an aromatic compound, so the reaction is also called the coupling reaction. Because of the relatively large size of the arenediazonium salt, the EAS reaction usually takes place at the para position due to steric hindrance.
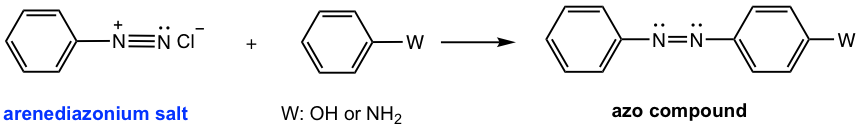
The mechanism for the coupling reaction (Fig. 5.5g) still follows the general mechanism of EAS, in which two steps are involved: the arenediazonium salt attacks the benzene ring as an electrophile, then deprotonation.
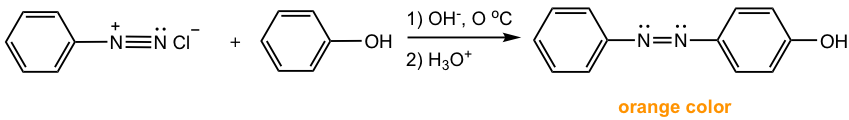
Reactions between arenediazonium salt and phenols are usually carried in a slightly alkaline solution. Phenol is present as phenoxide ArO– in such conditions, which is more electron-rich than phenol itself and more reactive towards EAS.
When arenediazonium salt reacts with aniline (or another aromatic amine), the reaction condition should be controlled as slightly acidic (pH 5 ~ 7). This is because that aniline will be protonated to unreactive aminium salt under a strong acidic condition and arenediazonium salt loses its reactivity in a strong basic condition.
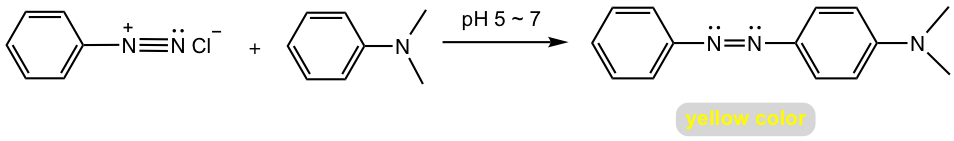
The azo linkage connects the two aromatic rings into an extended conjugation system, therefore azo compounds are usually intensely colored and are used as synthetic dyestuffs that are referred to as azo dyes. The product of the reaction in Fig. 5.4h used to be employed as a food dye called butter yellow.

