Chapter 5: More Reactions on Aromatic Compounds
5.3 EAS on Di- or Tri-substituted Benzenes
For EAS reaction on disubstituted benzene, the directing effects of both substituents have to be considered.
When both substituents direct the incoming reaction onto the same position, it is easy to predict the product, as in the example given below for o-hydroxybenzoic acid (Fig. 5.3a). The ortho and para position of the OH group is also the meta position of the COOH group, so the EAS of o-hydroxybenzoic acid occurs on the positions with orange arrows.
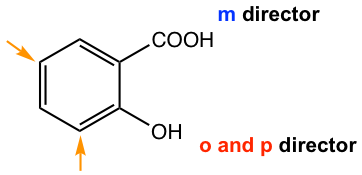
If the two substituents direct the incoming reaction on different positions, a stronger activating substituent wins out over a weak activating or a deactivating group.
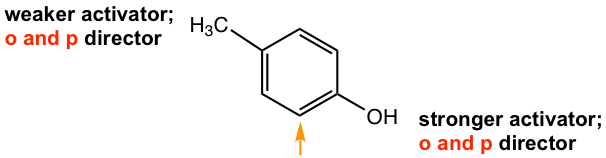
For the case of p-methylphenol (Fig. 5.3b), the ortho position of OH is the meta position of CH3. Since OH is the stronger activating group, it determines the position in which the coming EAS takes place, that is the position with the orange arrow.
The above guidelines also apply to tri-substituted benzenes.

