Chapter 5: More Reactions on Aromatic Compounds
5.1 Activating or Deactivating Effect of the Substituents on EAS
We have had thorough discussions about EAS reactions on the benzene rings in Chapter 4. EAS reaction also takes place on substituted benzenes, and the substituent already present on the benzene ring affects the position in which further EAS occurs, and the rate of the reaction.
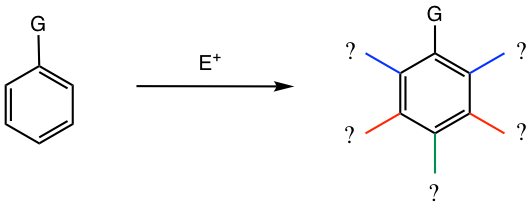
When substituted benzene undergoes EAS reactions, the substitute that is on the benzene ring has effects on two aspects, which will be discussed here and in section 5.2.
- Activating or deactivating effect: change the reactivity of the ring toward EAS;
- Directing effect: affects the orientation of the incoming group on the ring.
The activating group is the substituent that makes the ring more reactive than benzene towards EAS, i.e., it reacts faster.
- Electron-donating groups make the benzene ring more electron-rich and more reactive towards the electrophile, so electron-donating groups are activating groups.

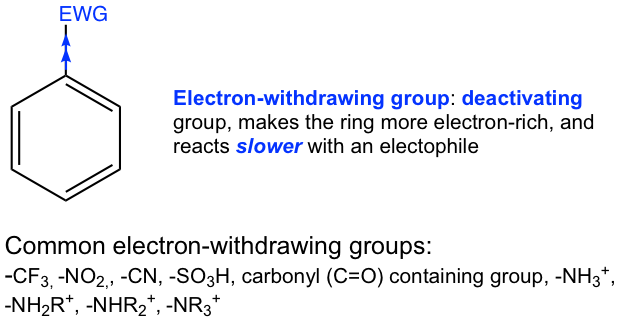
The deactivating group is the substituent that makes the ring less reactive than benzene towards EAS, i.e., reacts more slowly.
- Electron-withdrawing groups make the benzene ring electron-poor, and less reactive towards the electrophile, so electron-withdrawing groups are deactivating groups.
How to Identify a Group Is Electron-Donating or Electron-Withdrawing Group?
A quick hint to tell if a group is electron-donating or electron-withdrawing is to check whether the atom that connects directly to the benzene ring has lone-pair electrons.
The group that has lone-pair electrons on the atom attached directly to the benzene ring is an electron-donating group.
The group that does not have lone-pair electrons on the atom attached directly to the benzene ring is an electron-withdrawing group.
The above trends don’t apply to alkyl group (electron-donating) and halogens (electron-withdrawing).
The above trend provides a quick hint for us to “memorize” the properties of groups, however, understanding the actual reasoning behind them is more important.
Understanding the Electronic Property of a Group
Alkyl groups
Alkyl groups are electron-donating because of the hyperconjugation effect, the effect we have learned in section 7.4 in Book I. The orbitals of the sigma bonds in the alkyl group overlap partially with the p orbital of the sp2 carbon in the benzene ring, so the electron density of the ring increases. The hyperconjugation effect is a rather weak effect to donate electrons, so alkyl groups are weak electron-donating groups.
Groups with heteroatom that contain lone-pair electrons
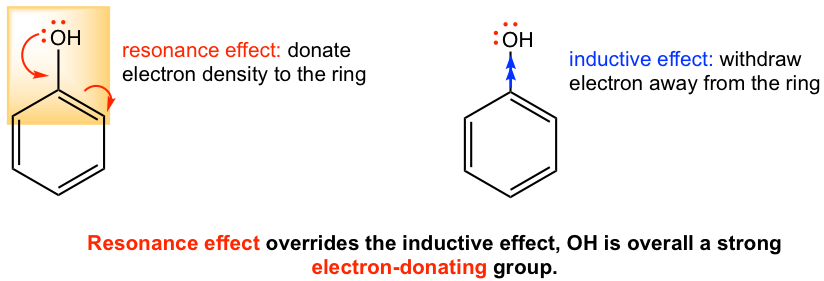
For groups with heteroatoms, most commonly N and O, there are two effects that work the opposite way. The inductive effect of the electronegative atom makes the group electron-withdrawing, while the resonance effect from the lone-pair electrons of the atom makes the group electron-donating. For most groups, the contribution through the resonance effect is stronger than the inductive effect, so most such groups (except halogens) are electron-donating. This is demonstrated in Fib. 5.1d with the OH group as an example.
Halogens
Detailed discussions on halogens will be given in section 5.2.
Groups with multiple bonds
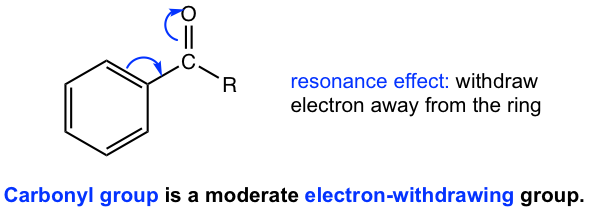
For substituents with multiple bonds, for example, -CN, -NO2, SO3H, and -C=O, resonance structures are available because of the multiple bonds. Such resonance effect works in the way of pulling electron density away from the benzene ring, therefore the groups with multiple bonds are electron-withdrawing. The example of the carbonyl group is shown in Fig. 5.1e.
Groups with Heteroatom that does not contain lone-pair electrons
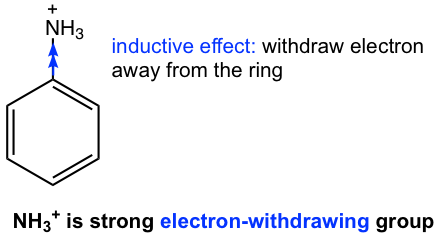
Inductive effect (because of the high electronegativity of the heteroatom, and positive charge for some groups) is the only effect involved for such groups since there are no lone-pair electrons available. Such groups are strongly electron-withdrawing groups, as the example of NH3+ in Fig. 5.1f.