Chapter 4: Aromatic Compounds
4.7 Friedel–Crafts Reactions
Friedel–Crafts reactions are also EAS. In the EAS reactions we learned so far, groups with heteroatoms (halogen, nitrogen, and sulfur) are introduced onto the benzene ring. Friedel–Crafts reactions are the methods to introduce carbon-containing groups onto the ring, as a result, they are of special interest in organic synthesis to build up larger molecules from smaller units like the benzene. They are so important that are discussed here in a separate section.
Friedel-Crafts reactions were discovered by Charles Friedel, a French scientist, and his collaborator American scientist James Crafts, in 1877. They are the methods for the preparations of alkylbenzenes (alkyl group, R) and acylbenzenes (acyl group, COR, or COAr), and are called Friedel–Crafts alkylation and Friedel–Crafts acylation respectively.
4.7.1 Friedel–Crafts Alkylation
The general equation for an alkylation reaction is shown here.
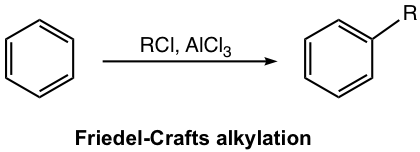
Aluminum chloride, AlCl3, also acts as the Lewis acid catalyst here (similar to the case of halogenation), to form a complex with an alkyl halide and generate carbocation that acts as an electrophile to react with the benzene ring. This is illustrated in the first step of the mechanism (Fig. 4.7b), the generation of electrophile.
Mechanism for Friedel–Crafts Alkylation
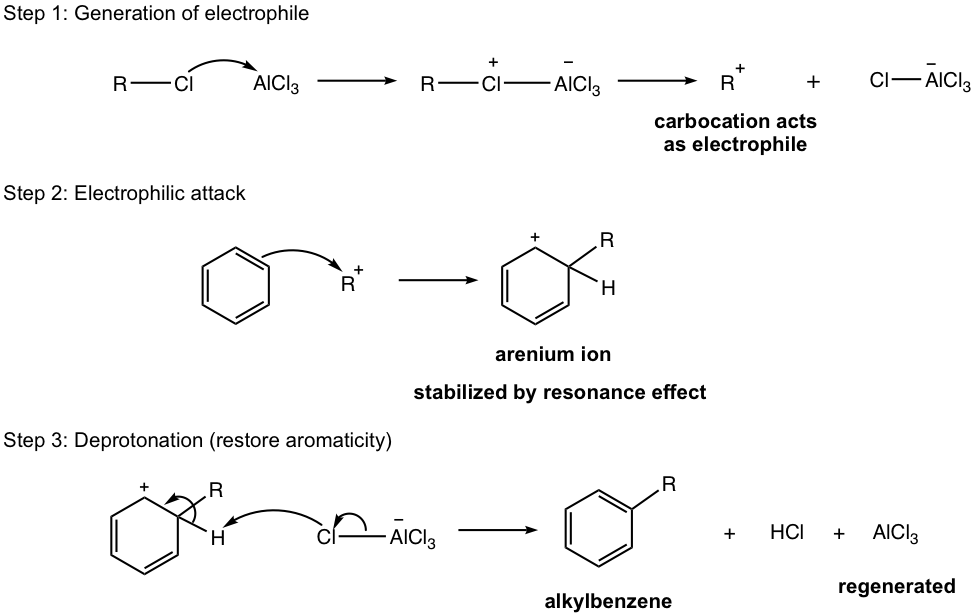
A couple of extra notes for the alkylation reaction:
- Other Lewis acids could also be used as catalysts, for example, BF3, SbCl5, and AlBr3.
- The reaction works well for primary, secondary, and tertiary halides. Since the primary carbocation does not form however because of the low stability, the complex of AlCl3 with RCH2Cl acts as the electrophile.
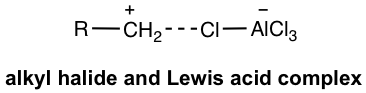
- Even though such a complex (in Fig. 4.7c) is not a real carbocation, it works so much like a carbocation and also undergoes carbocation rearrangement when available (reaction #3 in the following example). This brings a problem or limitation for the alkylation.
- Carbocation can be generated by other methods as well, for example by a mixture of alkene and an acid, or by dehydration of alcohol in the presence of acid, as shown in reactions #4 and #5 of the examples below.
- Another problem with alkylation is that polyalkylation might occur. Once an alkyl group is introduced into the benzene ring, it activates the ring towards further EAS because the alkyl group is electron-donating (further discussion on activating effect in Section 5.1).
Examples
Show the alkylation product.
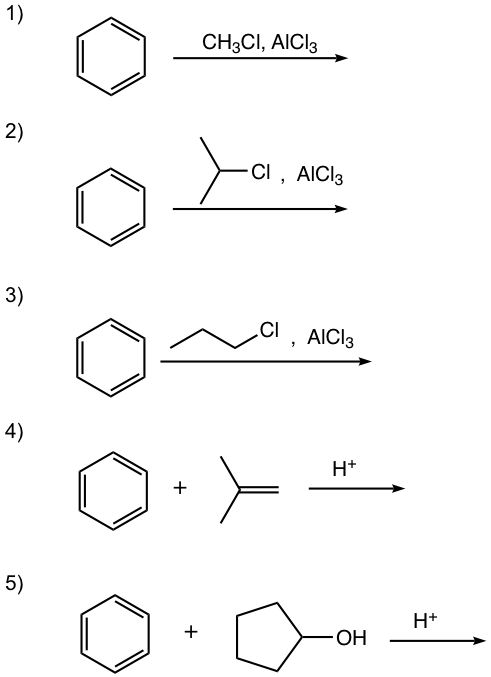
Answers:
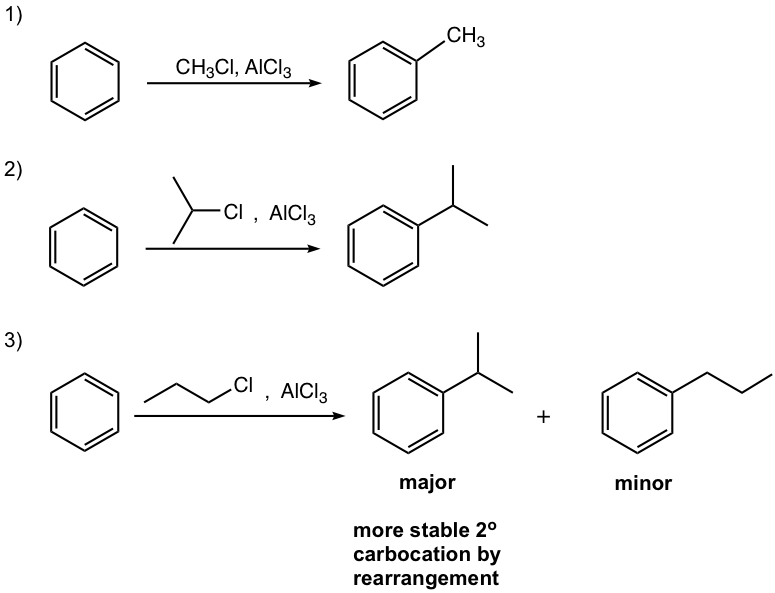
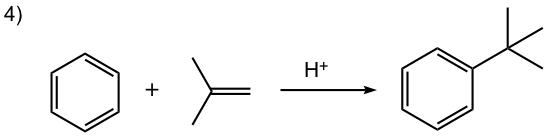
In this reaction, the carbocation intermediate is generated by the protonation of alkene, and then reacts with the benzene ring by EAS.
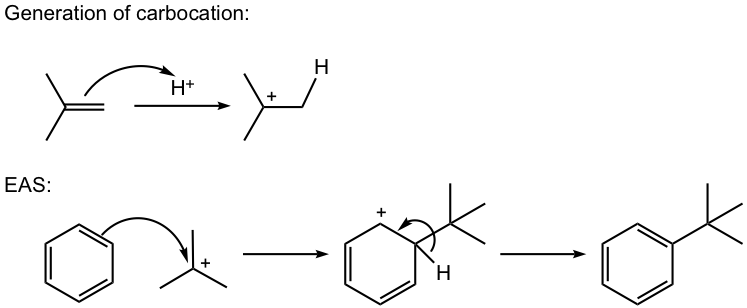
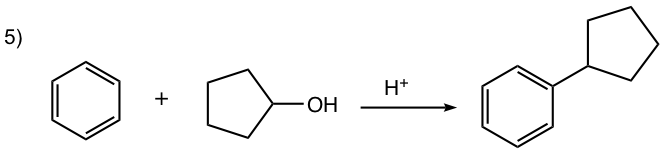
For this reaction, the carbocation intermediate is generated by dehydration of alcohol in the presence of acid, then reacts with the benzene ring by EAS.
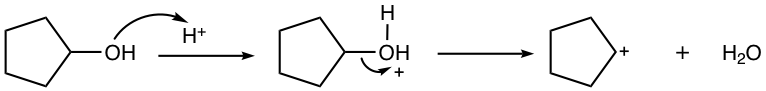
4.7.2 Friedel–Crafts Acylation
The acyl group (COR, or COAr) is introduced into the benzene ring in the acylation reaction. The reagent usually employed for this purpose is the mixture of Lewis acid and acyl halide, as shown in Fig. 4.7d. The detailed mechanism will be discussed next in Fig. 4.7e.
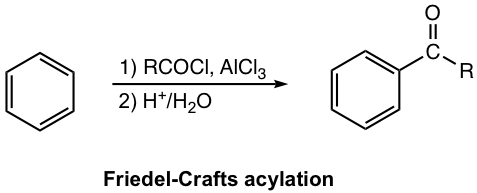
Mechanism for Friedel–Crafts Acylation

With the aid of Lewis acid, the halogen atom in acyl halide leaves and the acylium ion is generated in the first step. Acylium ion acts as an electrophile to react with the benzene ring. The following steps, steps 2 and 3, electrophilic attack and deprotonation, are standard in the EAS mechanism.
A special part of acylation is that two extra steps (steps 4 and 5) take place. After step 3, Lewis acid is still present in the reaction mixture, and it forms an adduct with ketone that acts as a Lewis base because of the lone pair electron on the oxygen atom. To get ketone as a final product, the work-up step with acidic water, therefore, is necessary to liberate the ketone from the adduct. For this reason, the two-step reaction consequences (shown in the general equation and examples next) are required for the acylation overall reaction condition.
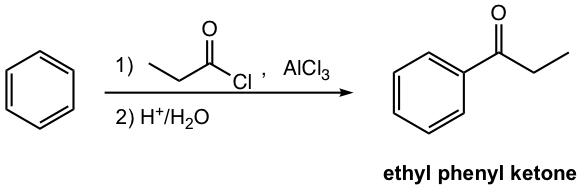
Please note a few other points for reagents used in the Friedel–Crafts acylation.
- Acyl chloride can be prepared by reacting carboxylic acid with thionyl chloride with heat.
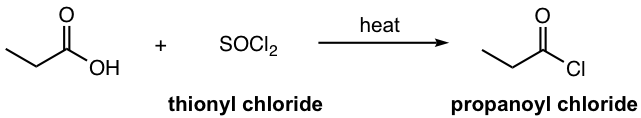
- Other than acyl halide, carboxylic acid anhydride can also be used for Friedel–Crafts acylation. Lewis acid is still required for anhydride, and the reaction mechanism is almost the same. The side product of using anhydride is the corresponding carboxylic acid.
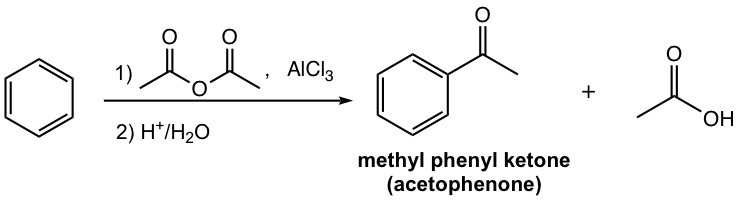
4.7.3 Application of Friedel-Crafts Acylation in Synthesis
Friedel-Crafts alkylation provides a way to introduce an alkyl group into the benzene ring directly, however, it has problems with rearrangement and polyalkylation as mentioned in section 4.7.1. So it is not an ideal method to synthesize mono-substituted benzene with an unbranched alkyl group.
Such problems don’t apply to acylation though. No rearrangement occurs for the acylium ion, the electrophile for acylation, since it is stabilized by the resonance effect. Furthermore, because of the deactivation of the product (refer to Section 5.1), the acylated benzene ring is no longer susceptible to further EAS, so no polyacylation. Therefore, if the acyl group is first introduced on the benzene ring, then reduced to the alkyl group, i.e., acylation + reduction could be a better alternate to synthesize unbranched alkylbenzene. This strategy has been proven successful and applied broadly in synthesis. The general equation is shown in Fig. 4.7i.
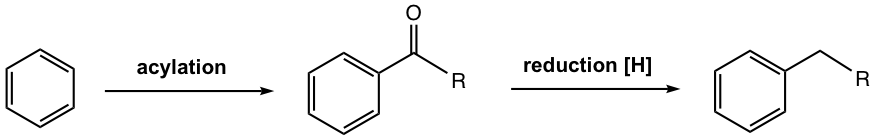
Reduction of Ketone to Methylene
Two methods are commonly applied to reduce the acyl group (ketone) to a methylene group.
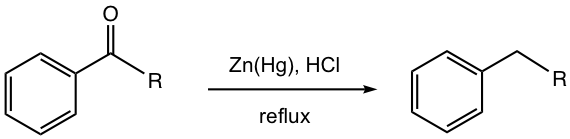
Clemmensen Reduction
The reducing agent used in Clemmensen reduction is the metal amalgam of zinc and mercury, with strong acid (concentrated HCl) and heat.
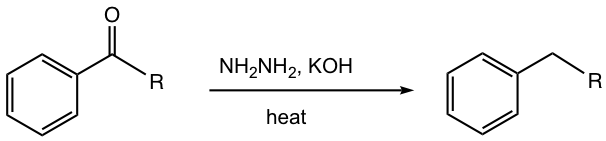
Wolff-Kishner Reduction
As we have learned in section 2.7.2, the hydrazone derivative of ketone can be further reduced to alkane when reacted in a strong base condition with KOH at a very high temperature.
These two methods are complementary to each other, with Wolff-Kishner reduction involving base, and acid needed in Clemmensen reduction.

