Chapter 4: Aromatic Compounds
4.6 Common EAS Reactions
4.6.1 Halogenation
Halogenation of benzene requires halogen (Cl2 or Br2) together with Iron (III) halide as a catalyst, for example, FeCl3 with Cl2, or FeBr3 with Br2 specifically. AlCl3 can be used for chlorination as well.
Iron (III) halide acts as Lewis acid in the mechanism, to make the halogen a stronger electrophile and facilitates the reaction. No halogenation reaction takes place for benzene without Lewis acid. The detailed mechanism is shown in Fig. 4.6b with bromination as an example.
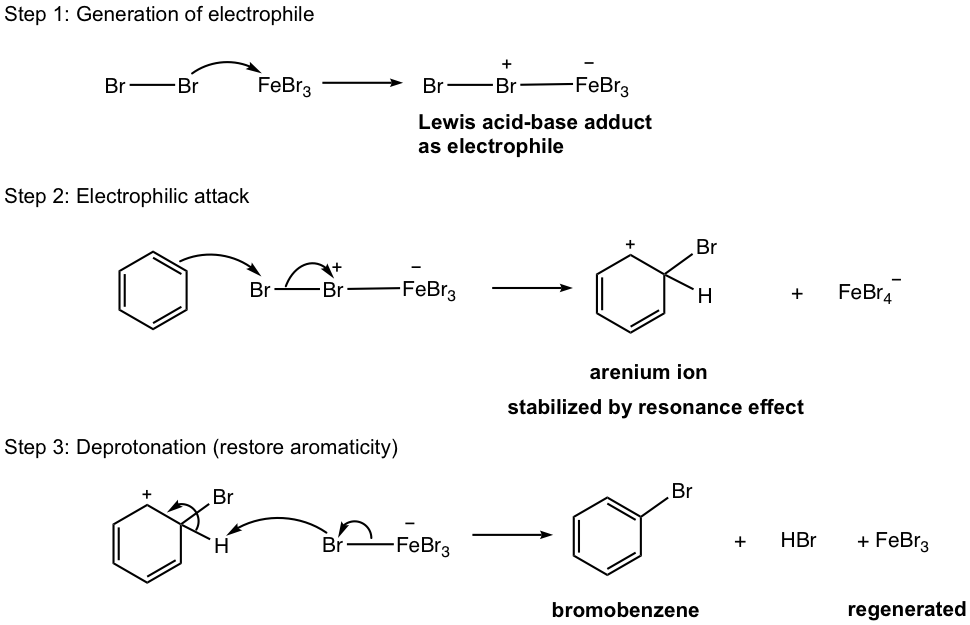
The bromine molecule is non-polar, so the bromine atom is not electrophilic enough to react with benzene directly. In the presence of Lewis acid FeBr3, the Lewis acid-base adduct is formed between FeBr3 and Br2. The Br-Br bond is polarized in the adduct and one bromine bears a positive charge, so it can react with benzene as a stronger electrophile.
While the generation of halogen electrophile is unique for halogenation, the following two steps are standard as shown in the general mechanism (Fig. 4.5d). Iron (III) bromide, the catalyst, is regenerated in the last step and can be reused in the reaction.
4.6.2 Nitration
Nitration reaction on benzene takes place with a mixture of concentrated nitric acid and concentrated sulfuric acid. Special attention is required to handle the concentrated acids mixture, which is a very strong corrosive oxidizing reagent.
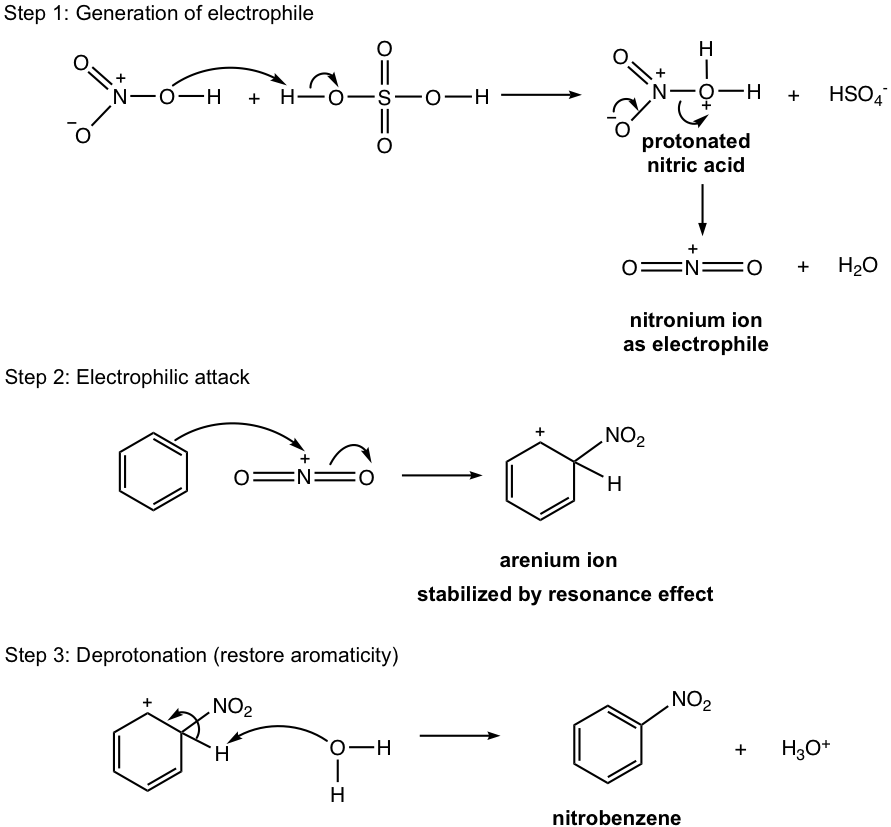
Again the most important and unique step for nitration mechanism is the generation of electrophile, NO2+, nitronium ion. In the first step, nitric acid is protonated by sulfuric acid (the stronger acid), and the protonated nitric acid dissociates to produce nitronium ion and water. Nitronium ion is the electrophile for nitration reaction to complete the following two standard steps, and produce nitrobenzene product.
4.6.3 Sulfonation
Benzene undergoes sulfonation when it reacts with fuming sulfuric acid. Fuming sulfuric acid is sulfuric acid with sulfur trioxide (SO3) gas dissolved. When sulfur trioxide is protonated by sulfuric acid, the SO3H+ cation is produced and acts as an electrophile for the sulfonation reaction, in which the benzenesulfonic acid is produced as a final product.
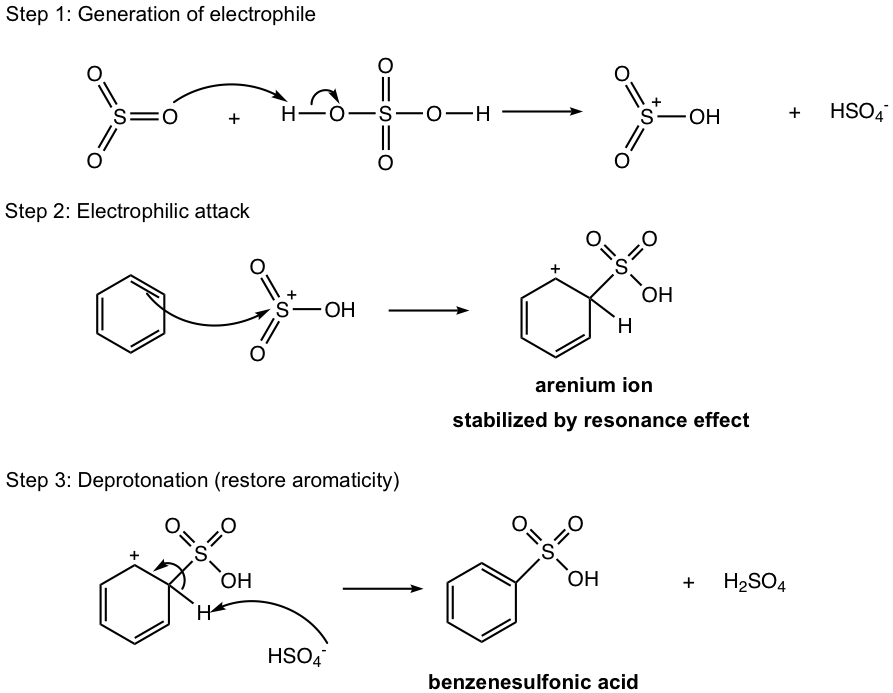
A special feature of this reaction is that the process is reversible, which means the benzenesulfonic acid can also be converted back to benzene. The position of the equilibrium depends on the reaction condition applied. Fuming sulfuric acid should be used to produce the sulfonation product, while the diluted sulfuric acid with heat allows the removal of the sulfonic acid group, or desulfonation, as illustrated in Fig. 4.6e.
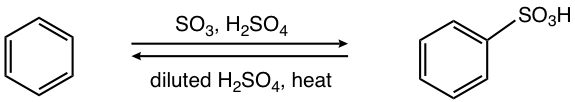
The reversibility of the sulfonation reaction makes the sulfonate group a suitable choice of the “blocking” group for the synthesis of benzene derivatives. The Sulfonate group is first introduced on the benzene ring to “block” a certain position, further substitutions occur next with other groups introduced onto other positions of the benzene ring, and the sulfonate group is removed at the end when no longer needed. Application of this strategy will be discussed in section 5.3.

