Chapter 3: Conjugated Unsaturated Systems
3.3 Conjugated Double Bond Systems
Structure and Naming of Dienes
A compound that contains two C=C double bonds is called diene, contains three C=C bonds is called triene. The following examples illustrate IUPAC naming for some simple dienes.

The two double bonds can be classified as cumulated, conjugated, or isolated depending on their relative positions.
- Cumulated: the two double bonds share one common carbon.
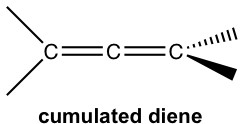
- Conjugated: the double bond and single bond alternate along the chain.
For conjugated diene, there are two possible conformations, the s-cis and the s-trans. The “cis” or “trans” here indicates the position of the two double bonds are either on the same or opposite side along the single bond in between. These two conformations are interconvertible through the rotation of the single bond for open-chain diene.

- Isolated: the two double bonds are separated by one or more sp3 carbons in between.
Conjugated dienes and conjugated polyenes are also conjugated systems. They will be the topic for this and coming sections, starting from 1,3-butadiene. We will focus on the special chemistry reactivities of these conjugated structures, and understand the reasons behind them.
Stability of 1,3-Butadiene and MO Theory
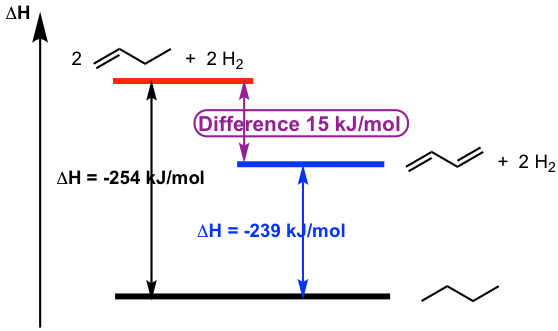
Similar to the allylic system, 1,3-butadiene also has special stability due to the conjugated structure. One proof of such stability arises from experimental data for the heat of hydrogenation.
The heat of hydrogenation is the heat released when the diene reacts with H2 gas to produce saturated alkane. The heat of hydrogenation of 1mol of 1,3-butadiene is -239 kJ/mol, and the heat of hydrogenation of 2mol of 1-butene is -254 kJ/mol. The energy difference of 15 kJ/mol is the evidence of stability difference between conjugated diene versus 2 moles of isolated alkenes.
The special stability of conjugated diene can also be explained by molecular orbital theory as shown in Fig. 3.3g. All four carbons in 1,3-butadiene are sp2 hybridized, each has an unhybridized 2p orbital. The four 2p orbitals combine to form a set of four π molecular orbitals.
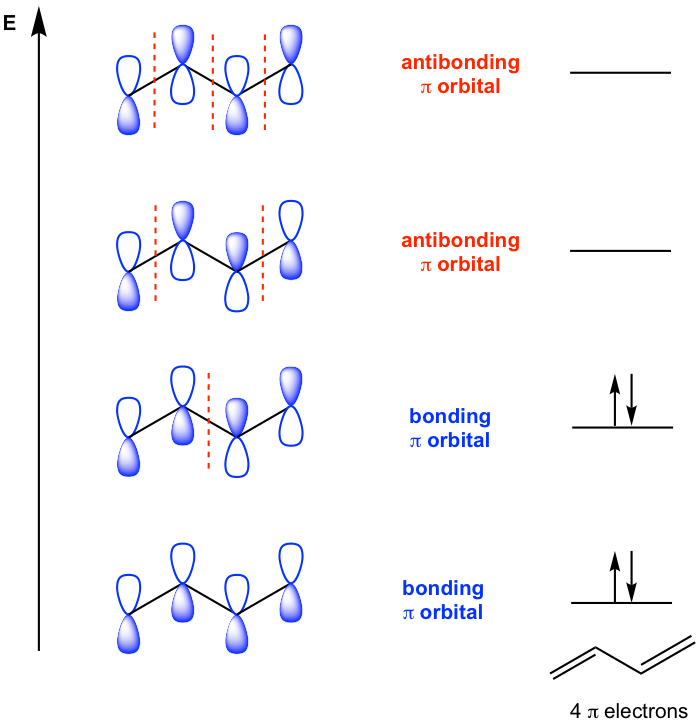
Two of the π molecular orbitals in 1,3-butadiene are bonding MOs, and the other two π molecular orbitals are antibonding MOs. The total four π electrons occupy the bonding orbitals in the ground state of 1,3-butadiene, which explains the special stability of the molecule. Such stability applies to all other conjugated polyenes as well.

