Chapter 2: Aldehydes and Ketones
2.8 The Wittig Reaction
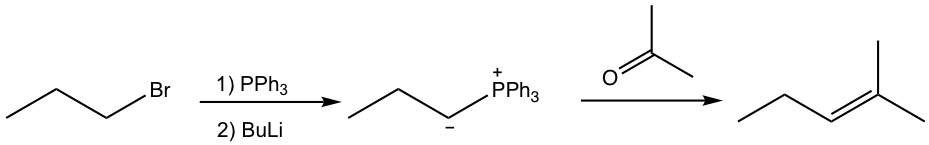
Wittig reaction is a type of interesting reaction with great applications. In this reaction, we will also encounter a new reagent which is called phosphorus ylide. This reaction is named for George Wittig who was awarded the Nobel prize for this work in 1979. The general equation of the Wittig reaction is shown below, with a specific example.
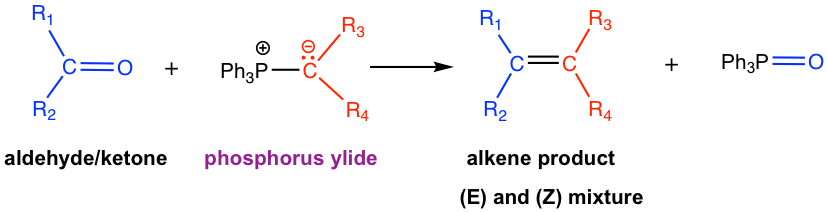
Wittig reaction provides a method for synthesizing alkene from aldehyde or ketone, by adding more carbons in the product. The product alkene can be regarded as an “add-up” by connecting the carbon-containing parts from both reactants together (blue plus red) via a double bond. Another great benefit of the Wittig reaction is that the location of the double bond is fixed, compared with other methods for the preparation of alkene, for example, E1, in which the mixture of constitutional isomers is obtained due to rearrangement. The reaction therefore can be designed by picking up the reactants that bear certain substituents that match the structure of the desired product.
The overall Wittig reaction involves two parts:
- Preparation of phosphorus ylide on site;
- Reaction of aldehyde or ketone with phosphorus ylide
The phosphorus ylide should be prepared freshly on site, without separation though. Freshly prepared ylide can then be mixed with carbonyl compound in the same reaction vessel, and the reaction can be accomplished in several hours. Therefore it is practically easy to carry out the Wittig reaction. Although practically it is not necessary to separate the phosphorus ylide, the overall reaction mechanism will still be discussed in two parts, for learning purposes.
Mechanism for the preparation of phosphorus ylide
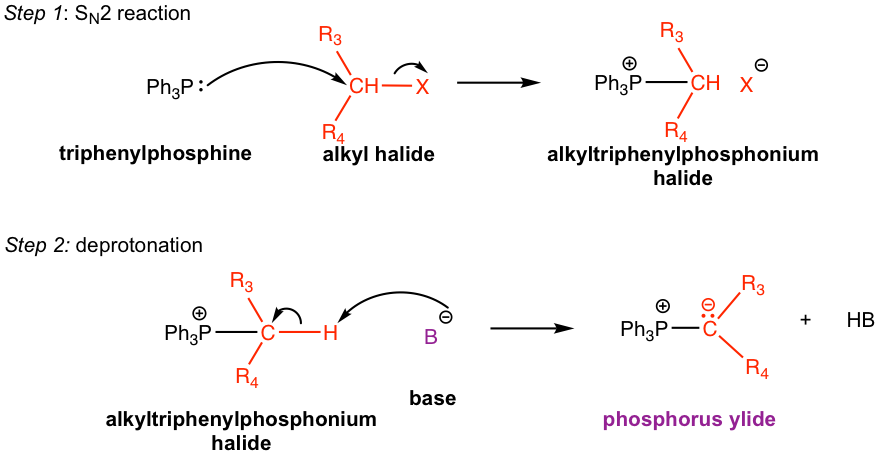
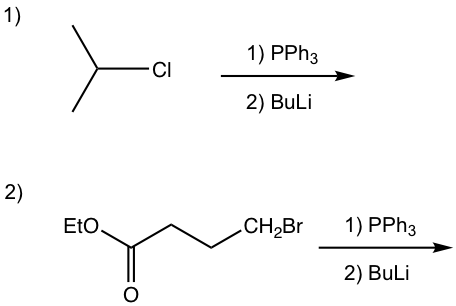
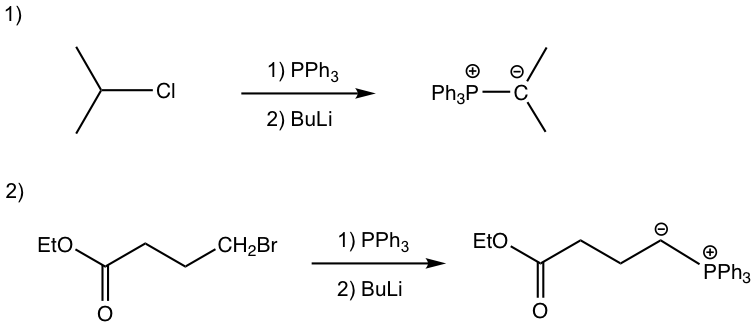
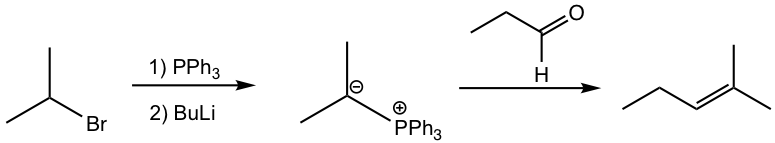
The preparation of phosphorus ylide involves two steps. The first step is a SN2 reaction, that the alkyl halide reacts with nucleophile triphenylphosphine (PPh3). PPh3, with the electron pair on the phosphorus element, is an excellent nucleophile due to the relatively large size of phosphorus and reacts readily with 1° and 2° alkyl halides by SN2 reaction. The SN2 reaction product, a phosphonium halide salt, is then deprotonated by a strong base in the second step to produce the phosphorus ylide. Organolithium compound and amide (NH2–) are usually used as bases in the second step. Even though the reaction proceeds with two steps, practically the alkyl halide, triphenylphosphine, and base can be mixed all together to prepare the phosphorus ylide.
Sometimes the resonance structure involves the C=P double bond is also used as the structure for phosphorus ylide. The quantum mechanical calculation indicates, however, that the charge separation structure is the major contributor.
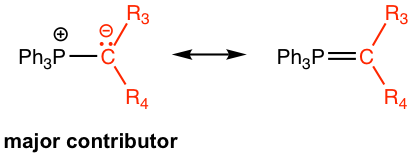
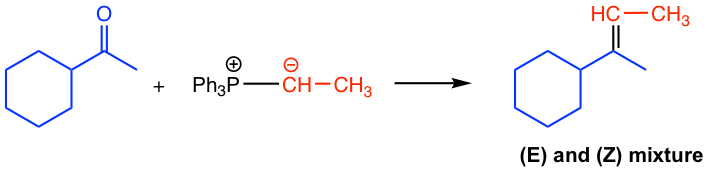
Examples
Mechanism for the Wittig reaction of phosphorus ylide with aldehyde/ketone
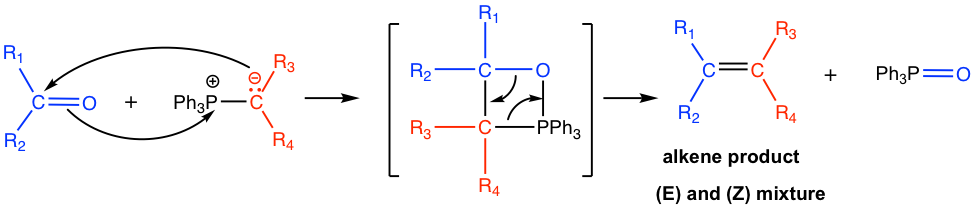
When phosphorus ylide reacts with the carbonyl group in aldehyde/ketone, a four-membered ring intermediate is first formed by the cycloaddition. Then the ring decomposes to form alkene and triphenylphosphine oxide, Ph3P=O. The formation of the very strong phosphorus-oxygen bond in Ph3P=O is the driving force for the reaction.
Examples
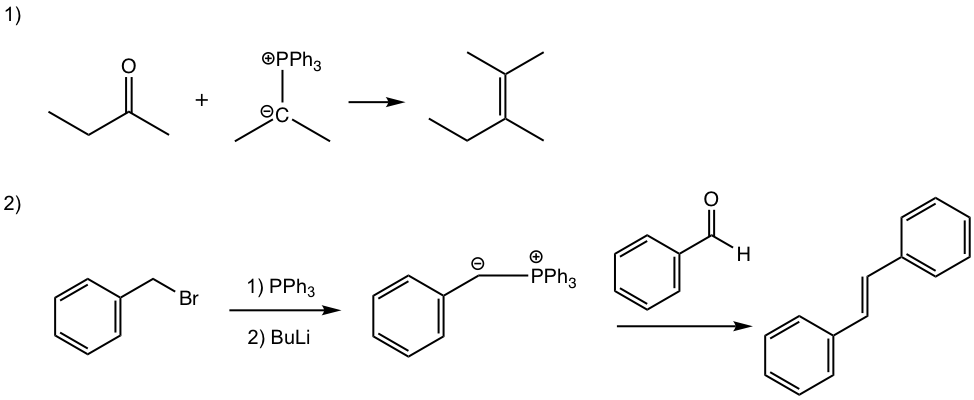
Fill up the product or the missing reactant/reagents for the following reactions.
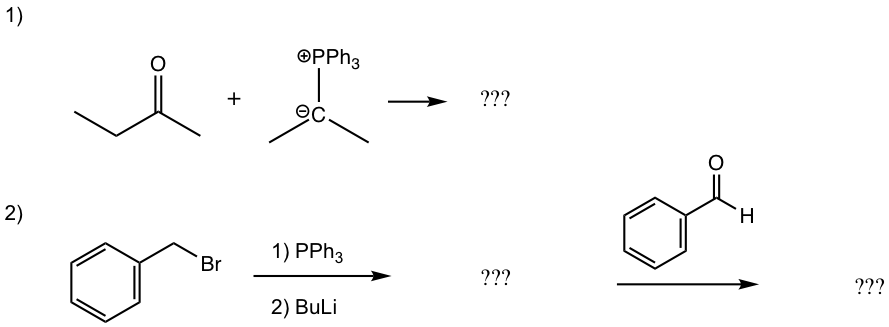
Answers:
Plan a Wittig Synthesis
Based on the structure of the desired alkene product, the synthesis route can be designed by choosing the proper aldehyde/ketone and alkyl halide for ylide.
Examples
Design synthesis of the following compound using a Wittig Reaction.
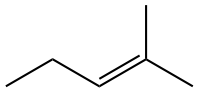
Analysis:
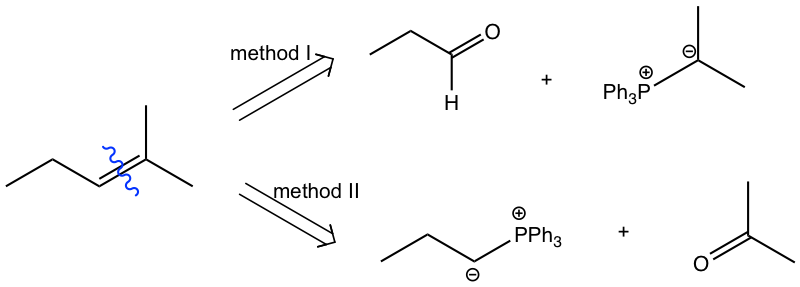
Method I:
Method II: