Chapter 2: Aldehydes and Ketones
2.7 Addition of Amines and Amine Derivatives to Aldehydes and Ketones
Amine is another type of compound that can do nucleophilic reactions with aldehydes and ketones. Depending on the type of amine reacted, different products are obtained, as summarized below.
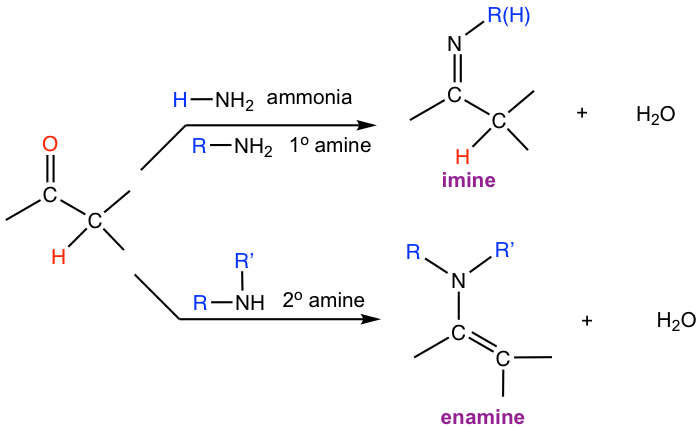
Ammonia or primary (1°) amines react with aldehydes and ketones to produce imines, the compound with a C=N double bond.
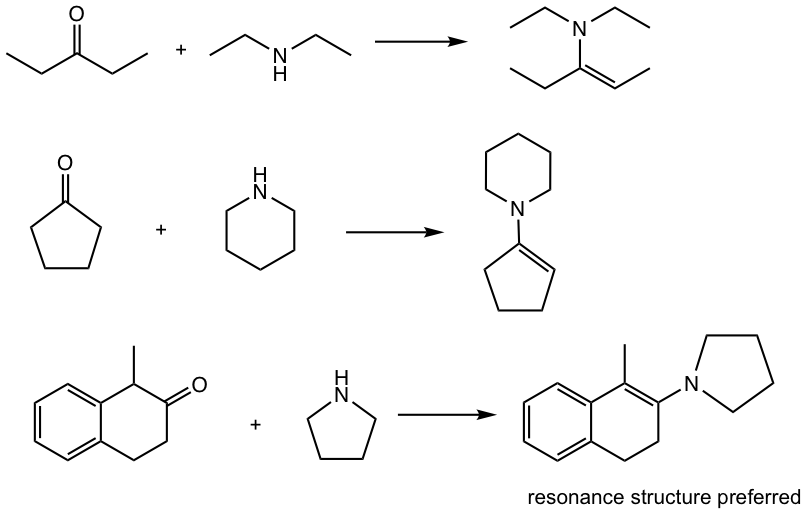
When secondary (2°) amines react with aldehydes and ketones, enamines are formed. Enamine means “alkenamine”, that is the compound contains the amino group beside a C=C bond.
2.7.1 Formation of Imines
The pH for reactions that form imine compounds must be carefully controlled in the range of 4 to 5. At low pH (strong acidic) conditions, most of the amine reactant will be protonated and become its ammonium salt, therefore losing the nucleophilicity. If the pH is too high on the other hand, there will not be enough acid (H+) present to protonate the aminoalcohol intermediate, a key step of converting the OH to the better leaving group H2O, therefore the reaction will not proceed.
The mechanism for the formation of imine is shown in Fig. 2.7b.
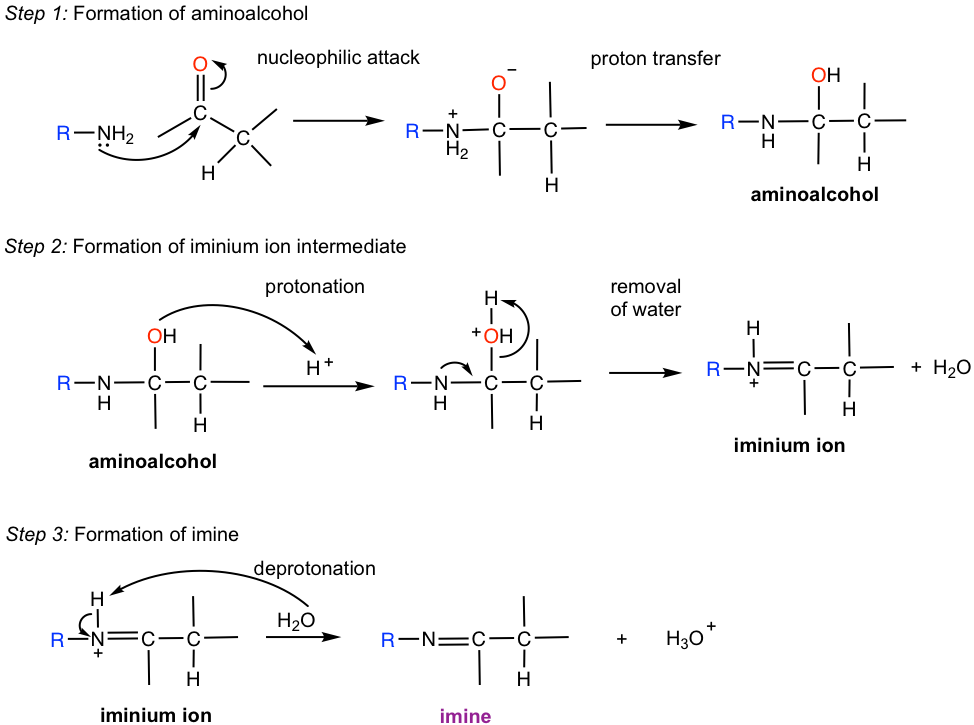
The overall mechanism for the formation of imine involves multiple steps. It is separated into “three steps” to make it easier to understand and memorize.
Step 1. Amine is the nucleophile and does a nucleophilic attack to the carbonyl carbon. The resulting intermediate is dipolar, which means N bears a positive charge and O bears a negative charge. A proton transfer then occurs intramolecularly to form a neutral intermediate aminoalcohol. Aminoalcohol can be regarded as the amine analog of acetal, that is NR(H) and OH groups bonded on the same carbon in aminoalcohol.
Step 2. The OH group in aminoalcohol is protonated, allowing it to leave as H2O. The iminium ion intermediated is formed after dehydration (removal of H2O).
Step 3. The H bonded on nitrogen is deprotonated to give the neutral imine product.
Hint: To figure out the structure of the imine product, replace the O atom in aldehyde or ketone with the N atom, and remember to keep the R group (or H for ammonia) in the amine reactant as well.
2.7.2 Imine Derivatives
The reaction between aldehyde/ketone and primary amines can be extended to a group of other compounds. The structures of these compounds all fit into the general formula of NH2-W (similar to the formula of primary amine). These compounds react with aldehyde/ketone in a similar way as primary amines do and form a series of products containing the C=N bond, that are called imine derivatives. The names and structures of the group of compounds, together with the imine derivative products are summarized in Table 2.2 below.
General Reaction

|
NH2—W |
Imine Derivatives |
|---|---|
| NH2OH hydroxyamine | 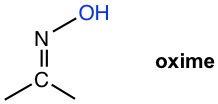 |
| NH2NH2 hydrazine | 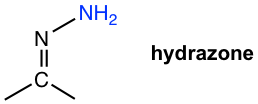 |
| NH2NHC6H5 phenylhydrazine | 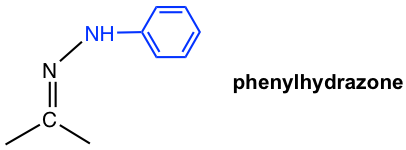 |
| NH2NHCONH2 semicarbazide | 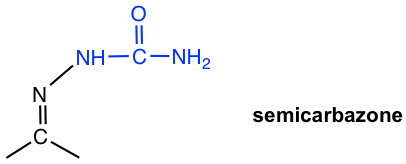 |
Table 2.2 Formation and Structures of Imine Derivatives
These imine derivatives are usually crystalline solids that have sharp and characteristic melting points. Such properties were used to be applied as a method to determine the identification of unknown carbonyl compounds. The melting point of the imine derivative of any known is determined and compared with the melting point of the same derivative of a known compound in a reference. Such a method was especially useful before the broad application of the spectroscopy technique.
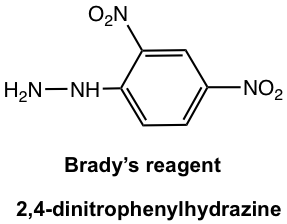
2,4-dinitrophenylhydrazine, a specific phenylhydrazine that is called Brady’s reagent, is still used as a reagent to detect the presence of aldehyde or ketone. The reaction of Brady’s reagent with aldehyde or ketone produces a bright precipitate of yellow, orange, or red color.
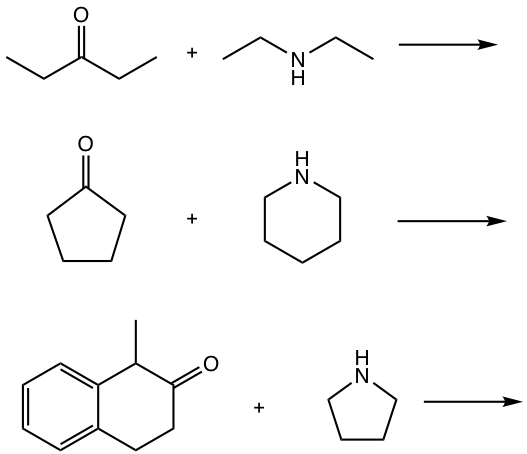
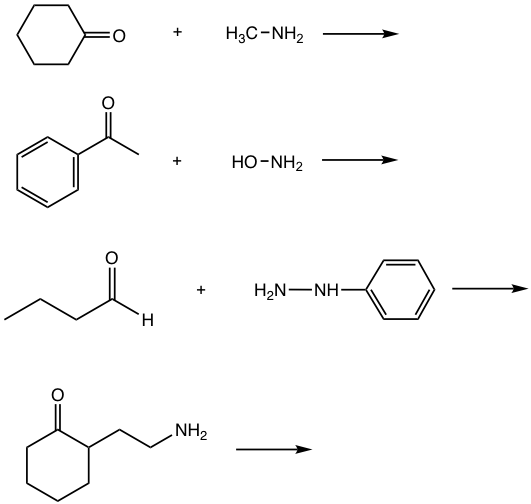
Examples
Show the product of imine or imine derivatives for the following reactions.
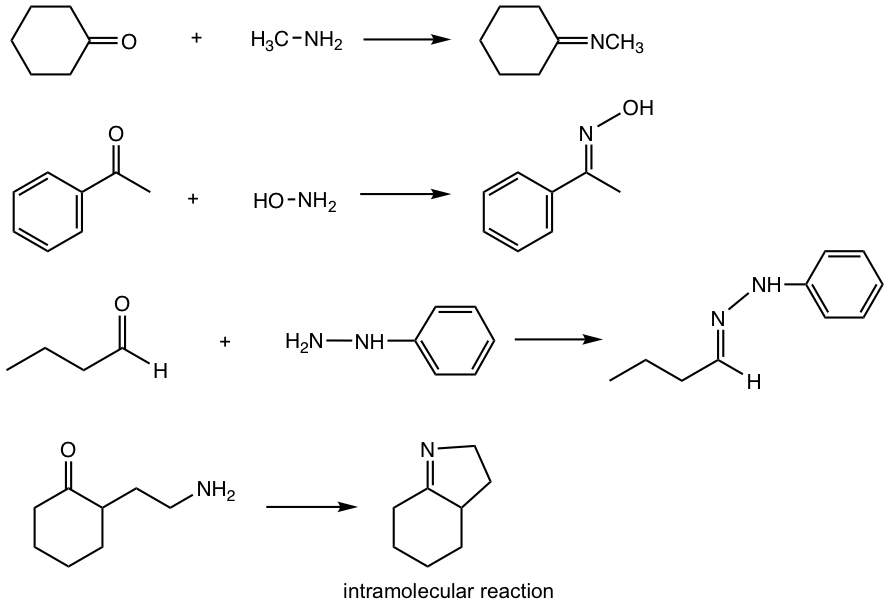
Answers:
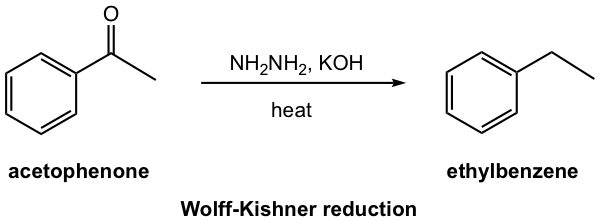
Wolff-Kishner Reduction
The hydrazone derivatives of ketones can be further reduced to alkane when reacted in a strong base condition with KOH at a very high temperature. The reaction is called Wolff-Kishner Reduction, a common method to do deoxygenation of the carbonyl group, and reduce ketone to alkane directly. The reaction proceeds well with the mixture of ketone, hydrazine, and base heated together. No isolation of the hydrazone intermediate is necessary.
2.7.3 Enamines
The reaction of secondary (2°) amines with the carbonyl group proceeds in the different mechanisms and forms enamine. The reason for the product difference is simply because there is one less hydrogen in 2° amines. See the mechanism in Fig. 2.7e for details.
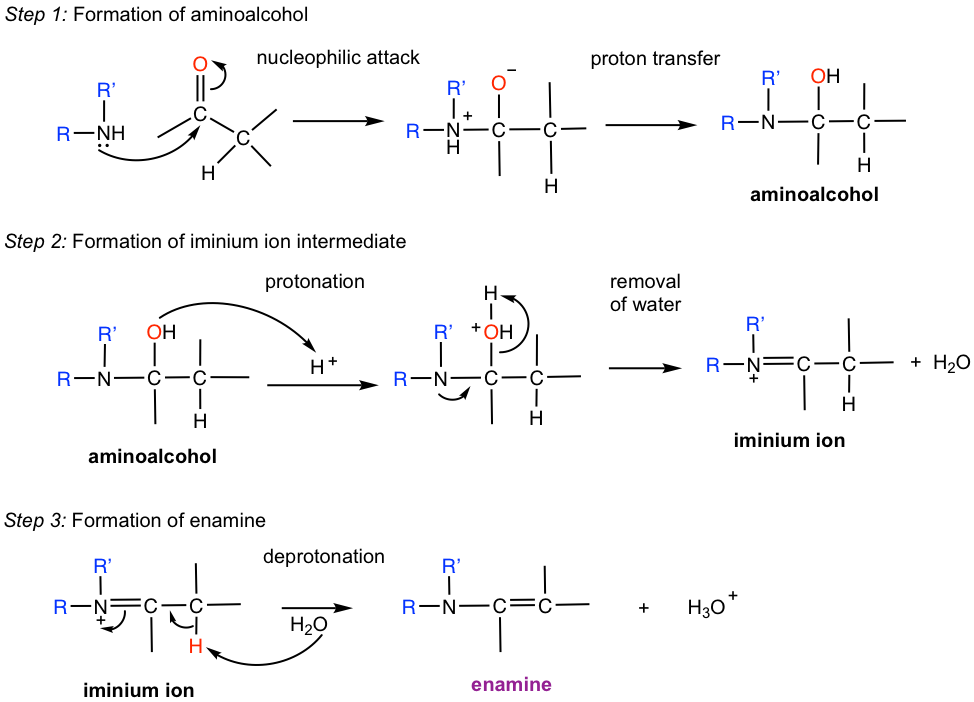
Comparison between the mechanism for the formation of imine (Fig. 2.7.b) and that of enamine reveals that the first two steps (up to the formation of iminium ion intermediate) are the same. The only difference lies in the last deprotonation step. Since there is no H available on the nitrogen atom for removal for 2°amine, the proton has to be removed from the adjacent carbon, resulting in the enamine.
Examples