Chapter 2: Aldehydes and Ketones
2.6 Protecting Groups in Synthesis
In organic synthesis, the strategy of applying a protection group is a very useful technique that has broad applications. The idea of protecting group is that if we would like a reaction to occur only on a “spot” selectively, then the other “spot” could be covered by a mask temporarily. The mask is called the protecting group.
A good choice of protecting group should meet a couple of criteria:
- Easy to put on;
- Unreactive or stable towards further reaction condition;
- Easy to remove
The acetal group, especially the cyclic acetal from diol is a good and common protecting group for aldehyde and ketone. Acetals satisfy all the requirements for good protecting groups:
- The reaction for the formation of acetal can be carried out conveniently, and the reaction occurs selectively with the carbonyl group in aldehyde/ketone.
- The acetal group is stable under basic conditions, that is the reaction condition for nucleophilic addition.
- The acetal group can be easily removed by acidic hydrolysis.
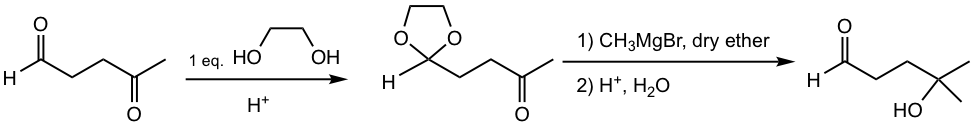
The following example demonstrates the application of protecting group in synthesis.
Examples
Propose the synthesis route for the following transformation.
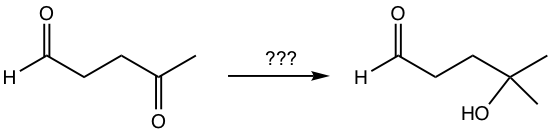
Analysis:
Grignard reagent is required for the conversion of ketone to alcohol. However, both aldehyde and ketone react with Grignard reagent, so the aldehyde should be protected to ensure the reaction occurs only on the ketone site. Because of the higher reactivity of aldehyde, with a limited amount of diol, aldehyde reacts first to get cyclic acetal, the protecting group.
Answer: