Chapter 2: Aldehydes and Ketones
2.3 Reactivities of Aldehydes and Ketones
In section 1.5.1, we have had a general discussion about the structure features and reactivities of the carbonyl group. More discussions will be needed here to understand the reaction diversities of this functional group.
2.3.1 Regions of Reactivity in Aldehydes and Ketones
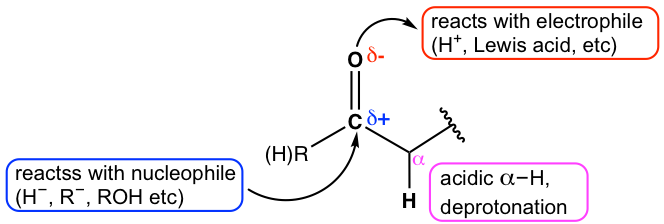
For carbonyl compounds, there are three reactivity centers as highlighted in Fig. 2.3a.
- The carbonyl carbon. Because of the polarity of the C=O bond, the carbon atom bears a partial positive charge, therefore it is electron-poor, and a great target for the attack by an electron-rich nucleophilic group. The reactions of metal hydrides (section 1.4.2) and organometallic reagents (for example Grignard reagent in section 1.4.4) with aldehydes and ketones are examples of this reactivity center. More reactions with a diversity of nucleophiles will be discussed in upcoming sections.
- The oxygen atom. For the same reason of C=O bond polarity, the oxygen atom bears a partial negative charge. Being an electron-rich spot, the oxygen atom is ready to react with other electrophiles, such as H+, or Lewis acid. Protonation is a common reaction for carbonyl oxygen when acid is present.
- The position that is adjacent to the carbonyl carbon, is called the α-position. Recall that the hydrogen atom at this position has a pKa of about 16~20 (section 3.3 in Book I). Deprotonation with a strong base readily occurs for α-hydrogen, and then triggers a series of interesting and important reactions. This will be the topic in Chapter 6.
The first two reactivity centers (carbon and oxygen atoms) will be the focus of this chapter. Because of the reactivity mentioned above, the most common and characteristic reaction of aldehyde and ketone is the addition of the carbonyl group with nucleophile, that is nucleophilic addition, the reactions we have learned in section 1.4.2 and 1.4.4 fit in this category. The general equation of nucleophilic addition for aldehydes and ketones is shown below.
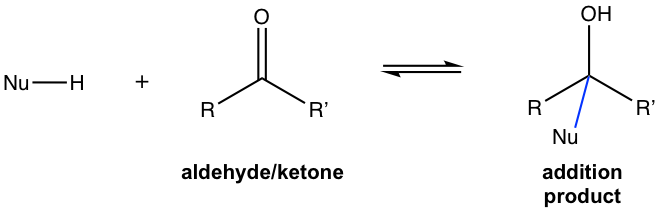
Depending on the strength of different nucleophiles, the detailed mechanism might be different, and there are generally two types of mechanisms for addition with strong nucleophiles (Fig. 2.3c) or weak nucleophiles (Fig. 2.3d) respectively. The mechanisms shown below are just for general purposes to help you gain the big picture of nucleophilic addition, the detailed steps and structure of products for different reactions still need specific attention.
Mechanism I — Nucleophilic addition with strong negatively charged nucleophile (Nu–):
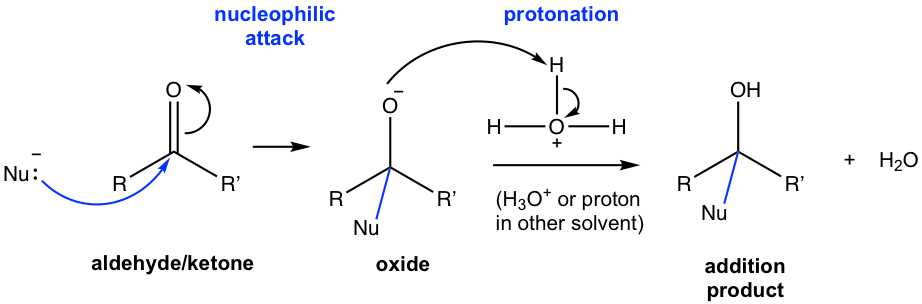
Because of the strong nucleophilicity of such nucleophiles, the nucleophilic attack occurs directly on carbonyl carbon in the first step, and a new bond is formed between the carbon and the nucleophile. A tetrahedral intermediate with full negative charge on oxygen (an oxide) is produced in this step. Such oxide anion, as a strong base, is susceptible to protonation with the solvent to give a neutral addition product. The overall addition involves two steps: nucleophilic attack, followed by protonation. The addition reactions of hydride (H–), organolithium reagent (RLi), and Grignard reagents (RMgX) (1.4.2 and 1.4.4) are typical examples of this category.
Mechanism II — Nucleophilic addition with weak nucleophile (NuH) and acid catalyst present:
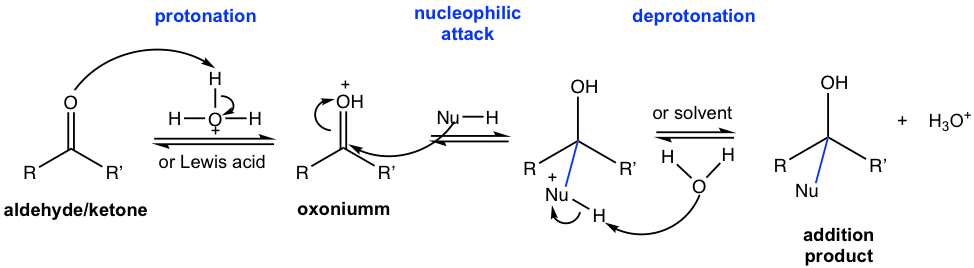
The carbonyl carbon, which bears only a partial positive charge, is not a strong electrophile. Therefore the ability of weaker nucleophiles, such as ROH and water, are not strong enough to do the attack directly. However, with catalytic acid present, the carbonyl group undergoes protonation first, and the protonated version of the carbonyl group, oxonium, exhibits stronger electrophilicity that is prone to react with weak nucleophiles. The overall addition therefore involves three steps: protonation, nucleophilic attack, and deprotonation.
Another point is that many nucleophilic additions to the carbonyl group are reversible. The structure and nature of reactants determine the overall results, or the position of the equilibrium.
2.3.2 Reactivity Difference between Aldehyde and Ketone
The structural difference between aldehyde and ketone is that there must be at least one hydrogen atom in the aldehyde, while ketone has two R groups. For steric hindrance, the larger R group makes it more crowded around the carbonyl carbon, and slows down the attack of the nucleophile. Furthermore, the e-donating effect of the R groups in ketone helps to stabilize the partial positive charge on the carbonyl carbon, and makes ketone to be less reactive towards nucleophilic attack.
As a result, generally, aldehydes are more reactive than ketones for nucleophilic addition.
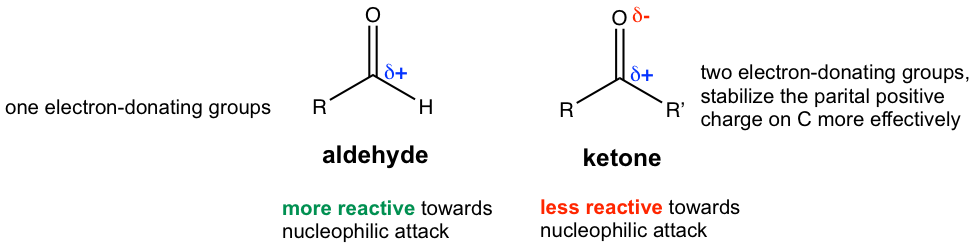
In upcoming sections (2.4 ~ 2.7), we will learn more specific reactions between aldehydes/ketones with different nucleophiles.

