Chapter 9: Carbohydrates
9.6 Disaccharide
Disaccharides are compounds that consist of two monosaccharide units that are connected by a glycoside linkage. Upon hydrolysis, disaccharide gives two monosaccharides.
9.6.1 Maltose
Let’s first take maltose as an example. Maltose is a disaccharide that could be obtained from the hydrolysis of starch, and is about 30% as sweet as sucrose (in next section 9.6.2). Maltose contains two D-glucose subunits that are connected by a glucosidic linkage between C-1 of one subunit and C-4 of the other unit, and the oxygen bonded to the anomeric carbon (C-1) is in the α-position. Such linkage can be named as α-(1→4)-glucosidic linkage. The structure is shown in Fig. 9.6a in both Haworth projection and chair conformation.
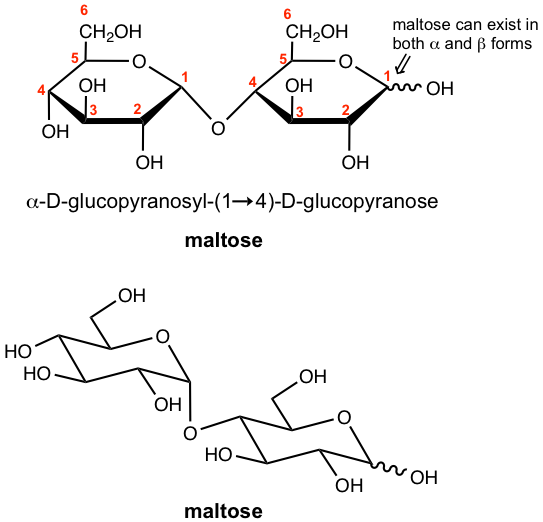
Maltose can exist in both α and β anomers, so the configuration of the anomeric carbon (the carbon with the hemiacetal group) on the right-hand ring is not specified and is marked with a wavy line. Also because maltose has the hemiacetal group in the right-hand subunit, and the hemiacetal is in equilibrium with the open-chain aldehyde and is easily oxidized, therefore it is a reducing sugar that gives positive tests with Benedict’s and Tollens’ reagents.
Examples
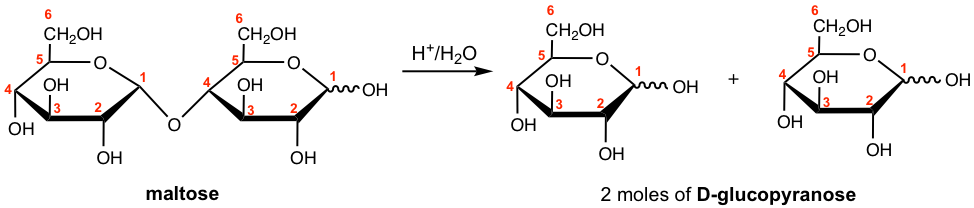
Show the hydrolysis product of maltose.
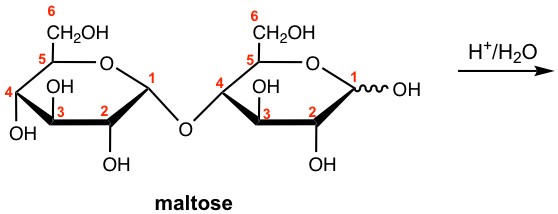
Answer: When 1 mole of maltose undergoes acid-hydrolyzed hydrolysis, it yields 2 mole of D-glucopyranose.
9.6.2 Sucrose
Sucrose is the most common disaccharide. It is the substance known as table sugar and is commercially obtained from sugarcane and sugar beets.
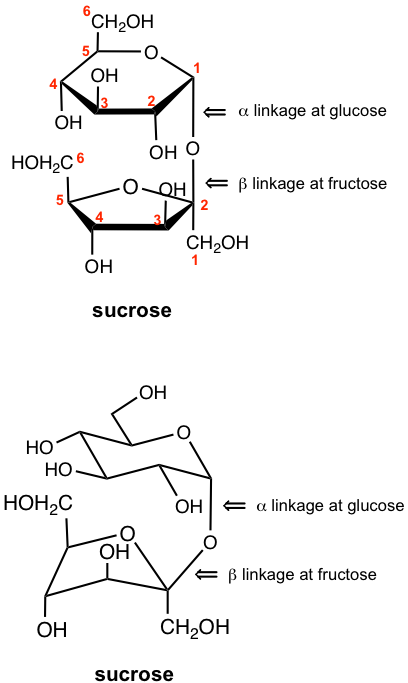
Sucrose consists of a D-glucose subunit and a D-fructose subunit connected by a glucosidic linkage between C-1 of glucose (in the α position) and C-2 of fructose (in the β position). It is unique among the common disaccharides in the way that the anomeric carbons of both glucose and fructose are tied up. Because the glucosidic bond of sucrose is between the anomeric carbon of both subunits, the molecule does not have a hemiacetal group, so it is not in equilibrium with open-chain form in an aqueous solution, and cannot be oxidized. Therefore, sucrose is a non-reducing sugar.
The hydrolysis of 1 mol of sucrose yields 1 mol of D-glucose and 1 mol of D-fructose. The hydrolysis can be catalyzed by either acid or certain enzymes. The fact that sucrose is only hydrolyzed by an α-glucosidase, an enzyme obtained from yeast, indicates the α configuration at the glucoside portion. The β configuration at the fructoside portion is also confirmed by the experimental result that sucrose is hydrolyzed by sucrase, an enzyme known to hydrolyze β-fructofuranosides.
9.6.3 Lactose
Lactose is a disaccharide found in milk. Lactose consists of a D-galactose subunit and a D-glucose subunit connected by a glycosidic linkage between C-1 of galactose (in the β position) and C-4 of glucose.
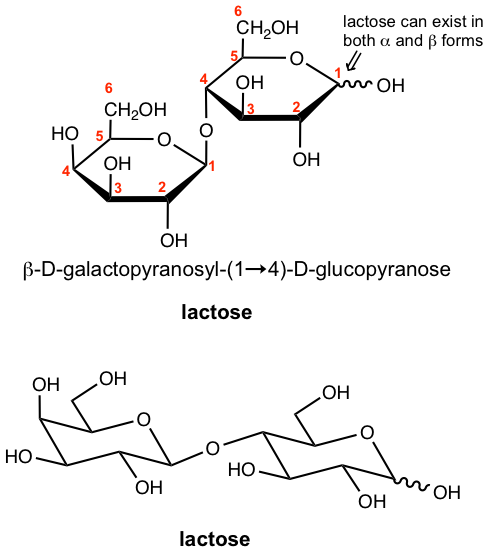
Lactose can also exist in both α and β anomers, similar to maltose, so the configuration of the anomeric carbon (with hemiacetal group) on the right-hand ring is not specified and marked with a wavy line. Also because there is a hemiacetal group in the glucose subunit of lactose, it is a reducing sugar.

