Chapter 9: Carbohydrates
9.4 Oxidation and Reduction Reactions of Monosaccharides
9.4.1 Oxidation with Bromine
Bromine, Br2, is a mild oxidizing agent and oxidizes the aldehyde group in the aldose to the carboxylic acid group. The resulting compound is an aldonic acid. The aqueous solution of bromine, Br2(aq), is used for this oxidization with a pH in the range of 5~6. No isomerization and fragment reaction for monosaccharides occurs in such mild acidic conditions.
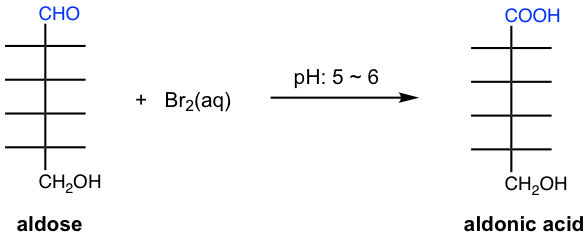
Ketoses are not oxidized by bromine water, as a result, this reaction can be used as a test to separate apart ketose and aldose. The disappearance of the reddish color for bromine water indicates the presence of aldose.
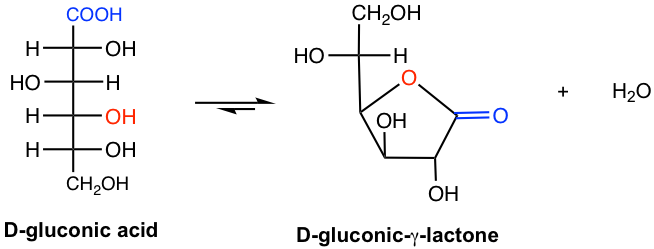
For aldose, the oxidation product aldonic acid may undergo a subsequent intramolecular esterification reaction, between COOH and the OH group on C-4, to form a γ-lactone, as shown in the following example.
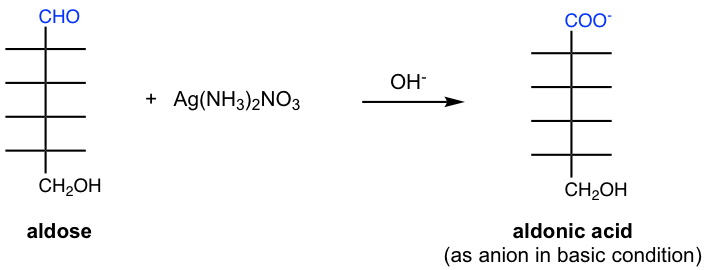
9.4.2 Oxidation with Tollens’ Reagent
Both aldoses and ketoses are oxidized to aldonic acids by Tollens’ reagent. Tollens reagent is prepared by mixing AgNO3 with aqueous ammonia solution and contains Ag(NH3)+ cation. Although the Tollens’ reagent does not react with the ketone group directly, ketose undergoes isomerization in an alkaline solution to aldose, which is then oxidized by the Tollens’ reagent.
Another oxidizing agent that contains Cu2+ is called Benedict’s reagent. Both aldoses and ketoses are oxidized by Benedict’s reagent as well, with a red precipitation of Cu2O generated. The appearance of the red precipitation is an indication of the positive result of the reaction.

Carbohydrates that give positive tests with Tollens’ reagent and Benedict’s reagent are known as reducing sugars.
- Aldoses, ketoses, and carbohydrates that contain a hemiacetal group are reducing sugars.
- Carbohydrates that contain only acetal groups don’t react with Tollens’ and Benedict’s reagents, and they are nonreducing sugars.
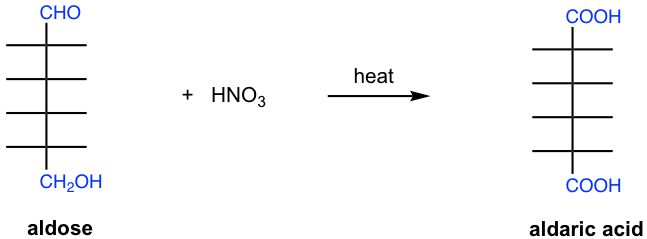
9.4.3 Oxidation with Nitric Acid HNO3
Dilute nitric acid is a stronger oxidizing agent and oxidizes both the aldehyde group and the terminal CH2OH group of an aldose to carboxylic acid groups, giving dicarboxylic acids that are known as aldaric acids.
9.4.4 Periodate Oxidation
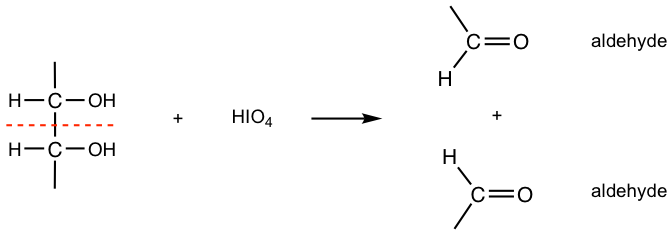
Periodic acid, HIO4, oxidizes compounds containing OH groups on adjacent carbons in a way called oxidative cleavage, that is, to break the C-C bonds and produce carbonyl compound products. We will examine the reaction with several types of structures involved.
- Two adjacent -CHOH groups react with one equivalent of HIO4, to generate two carbonyl compounds.
- Oxidative cleavage also takes place when an OH group is adjacent to the carbonyl group of an aldehyde or ketone.
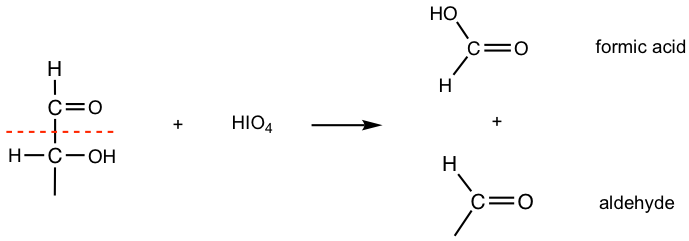
Please note that for every C-C bond cleavage, a C-O bond is formed at each carbon. With the extra C-O bond “added” onto the C=O carbonyl group, the carboxylic acid, COOH, is produced.
- When there are three or more contiguous -CHOH groups, the internal ones are obtained as carboxylic acid. This can be explained in the way that the internal carbon undergoes bond cleavage twice, it converts to C=O at first, then converts to COOH after the second time cleavage.
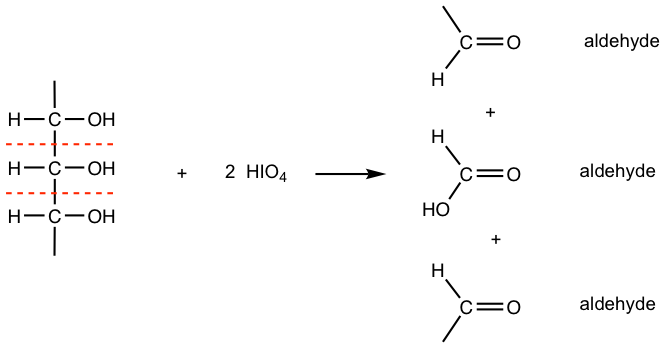
Please note that each C-C bond reacts with one equivalent of HIO4, therefore two equivalents of HIO4 are required for three contiguous -CHOH groups (or C=O groups), three equivalents of HIO4 are required for four contiguous -CHOH groups (or C=O groups), and so on.
Examples
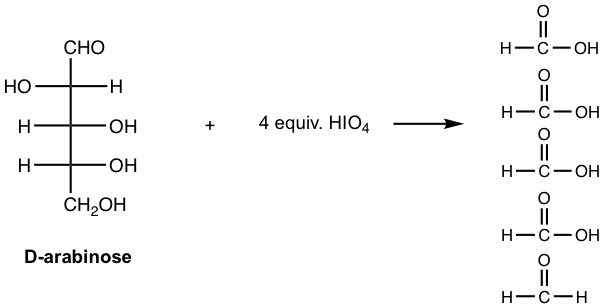
For the reaction of D-arabinose with HIO4, indicate the amounts of HIO4 required and show all the products.
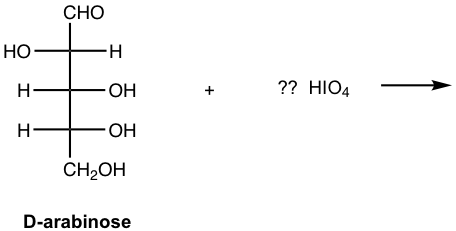
Answer:
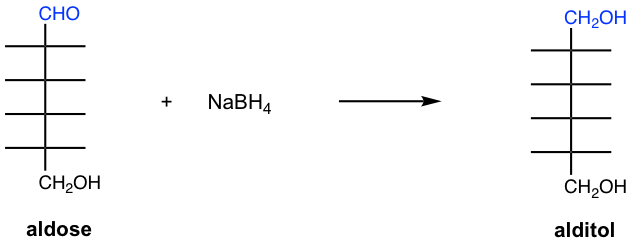
9.4.5 Reduction with NaBH4
The carbonyl group in aldoses and ketoses can be reduced by sodium borohydride, NaBH4, to produce polyalcohol which is known as alditol. Reduction of aldose forms one alditol product as shown in Fig. 9.4i.
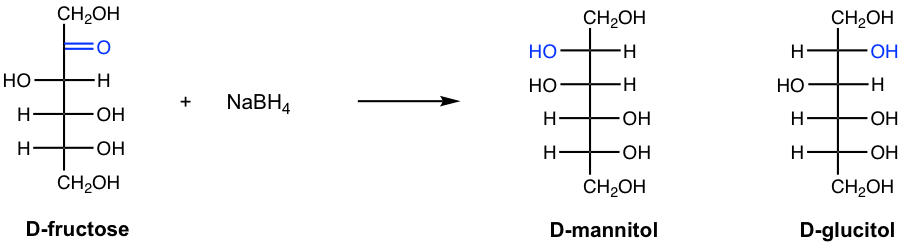
For the reduction of ketoses, however, two alditols are formed because the reduction creates a new chirality center and both stereoisomers are possible. The reduction of D-fructose is given as an example in Fig. 9.4j, in which both D-mannitol and D-Glucitol are produced.
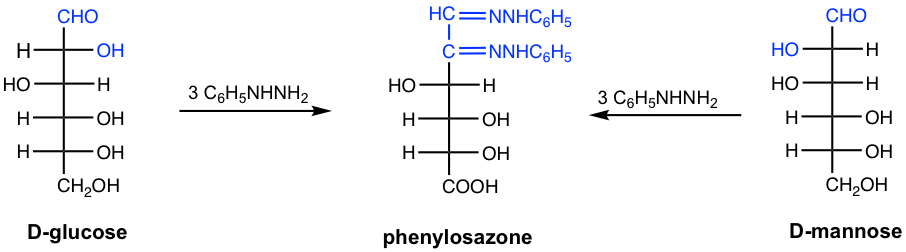
9.4.6 Osazones: Reaction of Monosaccharides with Phenylhydrazine
Since aldoses or ketoses have the carbonyl group, they also react with phenylhydrazine, but in a different way than that of common aldehyde or ketone. Aldoses and ketoses react with three equivalents of phenylhydrazine to produce a type of compound called osazones. The osazone derivatives of monosaccharides are crystalline solids that are insoluble in water, and could be easily isolated and purified, they were once used to identify monosaccharides.
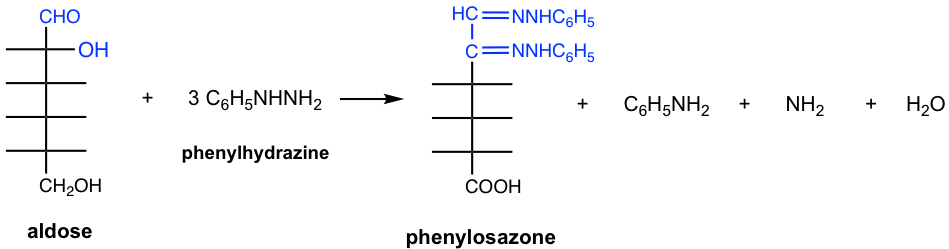
The simplified way to understand why three equivalents of phenylhydrazine react with aldose or ketose is that one equivalent oxidizes the OH group (the OH at C-2 for aldose, the OH at C-1 for ketose) to a carbonyl group, and two equivalents react with two carbonyl groups to form two imines. The actual mechanism is more complicated though.
Since C-2 in osazone is not chiral carbon, two aldoses that are epimers at C-2 form the identical osazones. For the example below, D-glucose and D-mannose, which are C-2 epimers, both produce the same osazone.