Chapter 8: The Claisen Condensation and Applications in Synthesis
8.3 β-dicarbonyl Compounds in Organic Synthesis
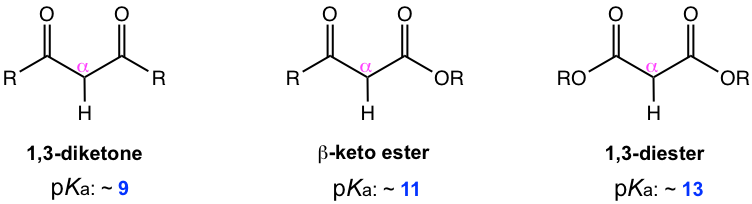
Depending on the structures of the reactant, different products could be generated in Claisen condensation, for example, β-keto ester, 1,3-diester, or 1,3-diketone, and they could all be generally categorized as β-dicarbonyl compounds. As mentioned in the earlier section the α-hydrogens in β-dicarbonyl compounds show great acidity (section 6.5.4, and in Fig. 8.3a), and therefore β-dicarbonyl compounds are important compounds that have unique applications in organic synthesis.
8.3.1 Alkylation of β-dicarbonyl compounds
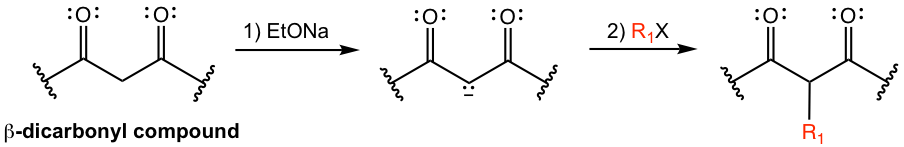
An enolate can be easily generated by treating a β-dicarbonyl compound with base, for example, alkoxide RO–. The resulting enolate, which is a nucleophile, undergoes alkylation with alkyl halide in an SN2 reaction. This reaction proceeds in the same way as the alkylation of other enolates (section 6.3).
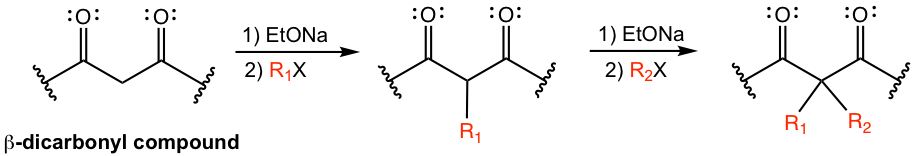
After the first alkyl group is introduced, there is still one acidic hydrogen available in the β-dicarbonyl compound, and if we desire, a second alkylation can be carried out. Two different alkyl groups can be introduced into the structure with two separate alkylation steps.
8.3.2 Decarboxylation of β-keto carboxylic acid
Decarboxylation is the reaction whereby a carboxylic acid loses a CO2 molecule. For most carboxylic acids, decarboxylation is difficult to be carried out. For β-keto carboxylic acid, that is the carboxylic acid with a carbonyl group one carbon away from the COOH group, decarboxylation occurs readily with heating.
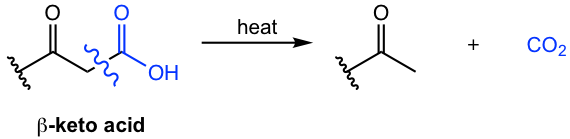
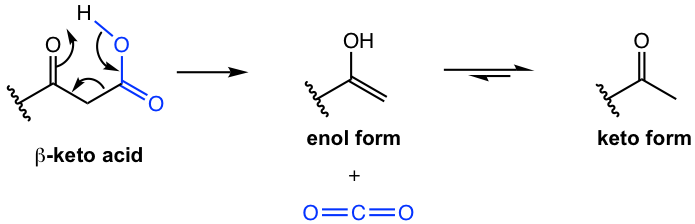
The reason for the ease of decarboxylation is that the carbonyl group at the β-position helps to form a six-membered cyclic transition state (Fig. 8.3e), that leads to the removal of the CO2 molecule and generation of an enol. The enol then tautomerizes to a ketone.
8.3.3 Application of Two Specific β-dicarbonyl Compounds in Synthesis
In organic synthesis, alkylation and decarboxylation are usually applied as subsequent steps to β-dicarbonyl compounds, to synthesize target products with specific structures. We will focus on the applications of two specific β-dicarbonyl compounds in this section, each represents a way for synthesizing one specific type of structure.
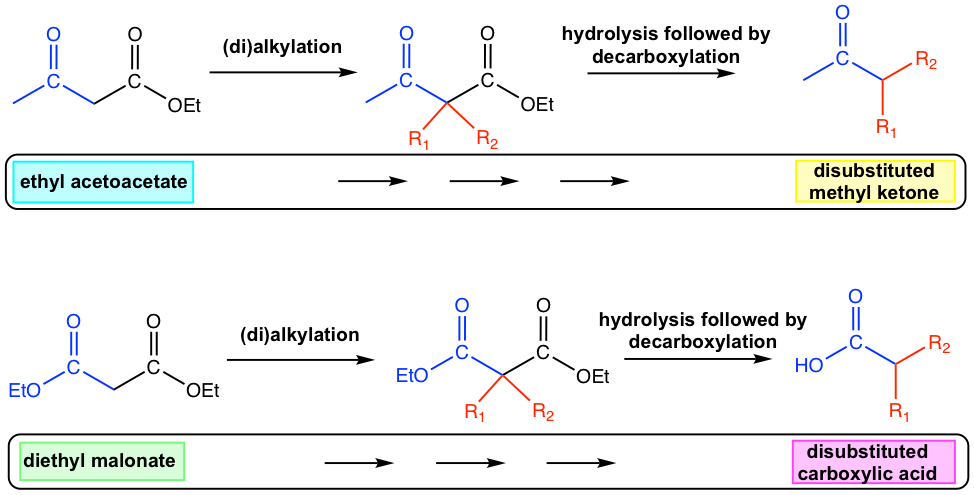
Acetoacetic Ester in Synthesis
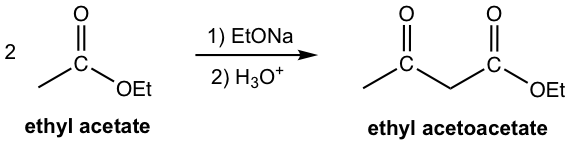
One specific compound that has a very common application in synthesis is ethyl acetoacetate. Ethyl acetoacetate can be prepared by self-Claisen condensation of ethyl acetate.
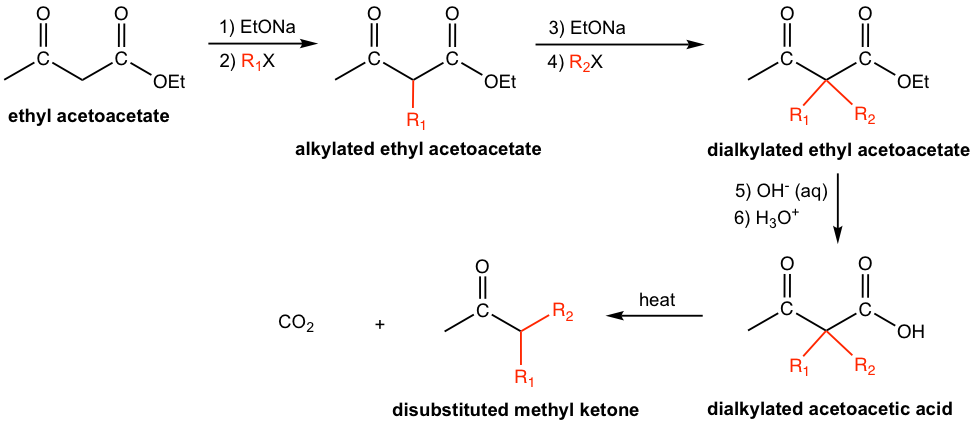
Upon alkylation, followed by hydrolysis and decarboxylation, ethyl acetoacetate is a useful reactant for the preparation of substituted methyl ketones (Fig 8.3g). Based on the structure of the target methyl ketone, i.e., the specific structure of R1 and R2, the corresponding alkyl halide can be employed either once or twice. The alkylated ethyl acetoacetate then be hydrolyzed to the alkylated acetoacetic acid, which undergoes decarboxylation with heating to produce the desired substituted methyl ketones.
Examples
Show products of the following multi-step reaction.
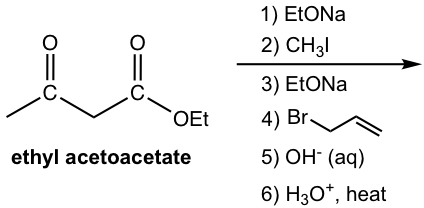
Answers:
All the steps can be put together on the reaction arrows. Only the final product is required for this question, the products for the middle steps are shown for study purposes.
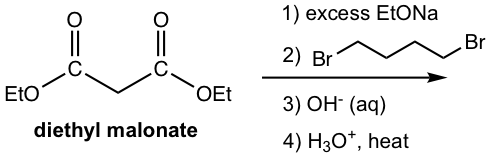
Malonic Ester in Synthesis
Another group of b-dicarbonyl compounds that has broad application in synthesis is the ester of malonic acid, with the specific example as diethyl malonate (or dimethyl malonate, which can be used in almost exactly the same way).
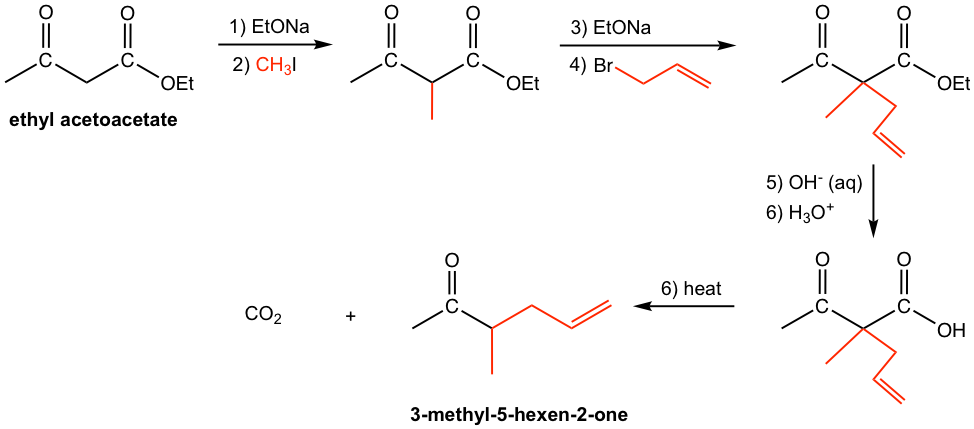
Diethyl malonate is prepared from the cross-Claisen condensation between diethyl carbonate and ethyl acetate.
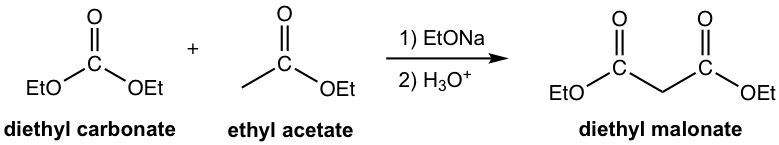
Applying the same synthesis strategy of alkylation, hydrolysis and decarboxylation, diethyl malonate is a useful reactant for the preparation of substituted carboxylic acid, as shown in the general equation here.
Examples
Show the product.
Answer:
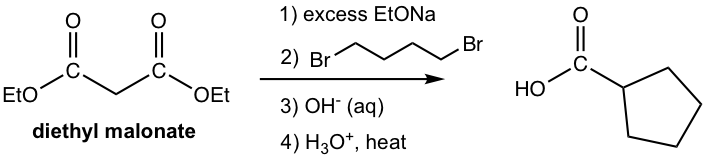
The synthesis applications of ethyl acetoacetate and diethyl malonate are summarized in Fig. 8.3j.