Chapter 7: Carboxylic Acid and Derivatives
7.5 Esters
7.5.1 Preparation of Ester
Preparation of Ester from Carboxylic Acid
Esters can be prepared through the reaction between carboxylic acids and alcohols, and the reaction is called esterification. Since carboxylic acid is not very reactive towards the acyl substitution, acid is required as a catalyst for esterification.
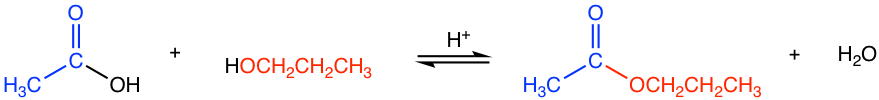
The reaction is reversible. Strategies such as using excess acid or alcohol, and removing water from the reaction mixture help to increase the yield of the ester.
The mechanism for acid-catalyzed esterification, which is shown in Fig. 7.5b, still generally follows the nucleophilic addition-elimination process, with a few extra proton-transfer steps though.
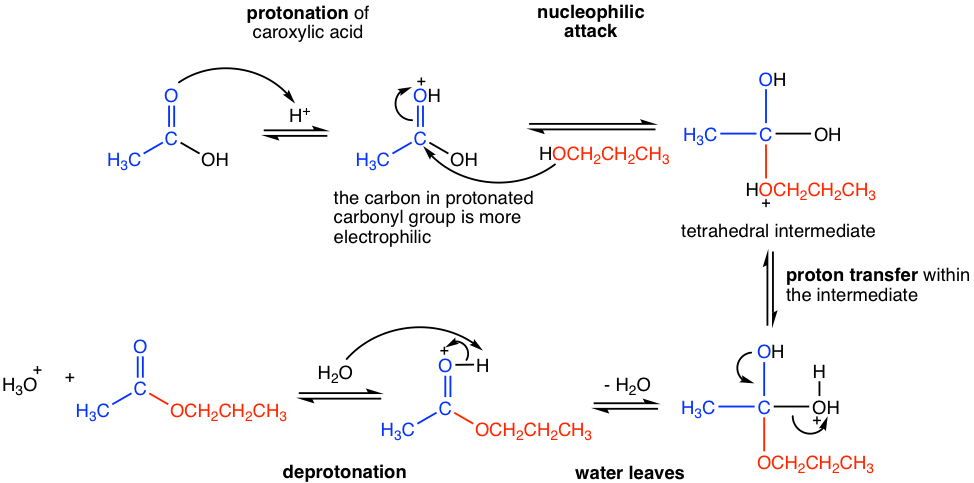
In the presence of strong acid, the carbonyl group in carboxylic acid is protonated. The carbon of the protonated carbonyl group is more electron-poor (or more electrophilic) and, therefore has higher reactivity towards nucleophilic attack. The neutral nucleophile, alcohol, then attacks the protonated carbonyl group and generates the tetrahedral intermediate. A proton transfer occurs next between the ether oxygen and the alcohol oxygen in the tetrahedral intermediate, then water leaves as the leaving group. A last deprotonation step gives the final neutral ester product. It is also indicated from the mechanism that the OR group (red part in the mechanism) in the ester comes from alcohol, and the acyl group (blue part in the mechanism) is from the carboxylic acid.
The reverse process of esterification is the acid-catalyzed hydrolysis of ester to produce carboxylic acid and alcohol back. See more discussions about hydrolysis in the next section 7.7.
Preparation of Ester from Acyl Chloride or Anhydride
As mentioned earlier both acyl chlorides and anhydrides, the more reactive derivatives, can be employed as starting materials to produce esters (refer to Fig 7.4b and Fig 7.4f). A few examples are shown below.
Examples
Show the product of the following reactions.
Answers:
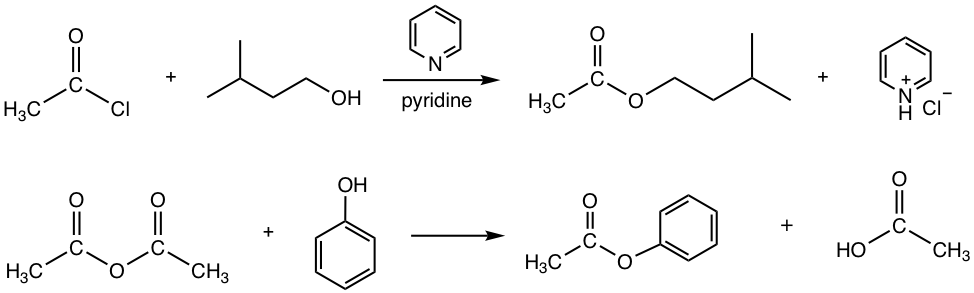
Preparation of Ester by Trans-esterification
An ester reacts with an alcohol R”OH (the R” in alcohol is different from the R’ in OR’ of the ester) under acidic conditions to form a new ester and a new alcohol. This reaction is called a trans-esterification because one ester is converted to another ester. Since the reaction is reversible, strategies that help to shift the equilibrium to the product side should be applied to ensure a high yield of the product, for example, by using an excess of reactant alcohol and removing the product alcohol from the reaction system.
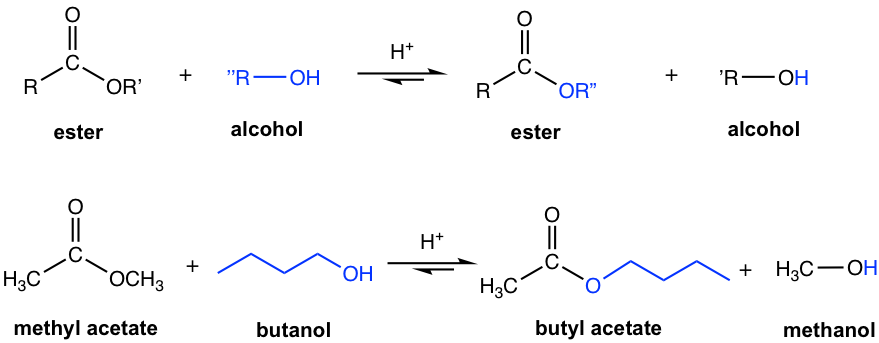
The mechanism of the acid-catalyzed trans-esterification is almost the same as the acid-catalyzed esterification of carboxylic acid. Both acid and ester are not very reactive towards acyl substitution (nucleophilic addition-elimination), protonation with acid helps to increase the electrophilicity of carbon in the carbonyl group and, therefore facilitates the reaction.
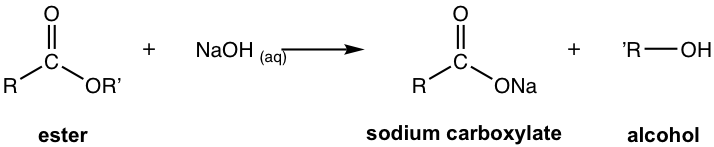
7.5.2 Saponification: Base-Promoted Hydrolysis of Ester
Base-promoted hydrolysis of ester is also called saponification, which is the chemical reaction that acts as the foundation of soap-making.
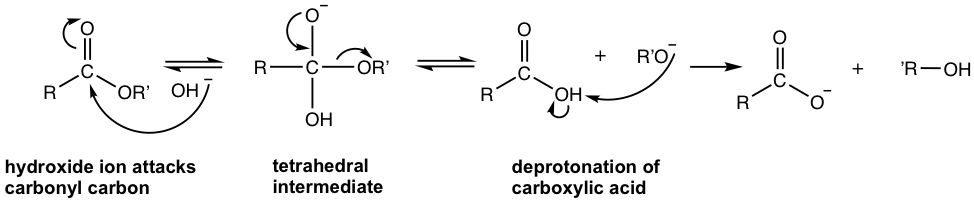
The base, OH–, increases the rate of the hydrolysis of the ester significantly because it is a stronger nucleophile than H2O, and attacks the carbonyl carbon readily. The product, carboxylate ion, is very unreactive toward nucleophilic substitution because of the negative charge it possesses. The base-promoted hydrolysis reaction, as a result, is irreversible. The mechanism is shown below.
The chemical structures of soaps are sodium (or potassium) salts of fatty acids. Fatty acids are carboxylic acids with long and unbranched alkyl groups (the length of the R group is usually longer than 16 carbons). Since these acids are obtained from fats, they are called fatty acids. The structures of fats are the triesters of fatty acids with glycerol, the alcohol with three OH groups. Upon base-promoted hydrolysis of the fats (triesters), fats are converted to the sodium (or potassium) salts of fatty acids, which is the soap. The commercial process of soap-making usually involves boiling of fats or oils in sodium hydroxide solutions, and the salt of fatty acid is then precipitated, dried, and pressed into the soap bars.


