Chapter 7: Carboxylic Acid and Derivatives
7.4 Acyl Chloride and Carboxylic Acid Anhydrides
7.4.1 Preparations of Acyl Halides
Acyl halides are the most reactive of the carboxylic acid derivatives, as a result, they cannot be prepared from any other derivatives. To prepare acyl halides, other special reagents will be needed. Thionyl chloride (SOCl2), phosphorus trichloride (PCl3), and phosphorus tribromide (PBr3) are the reagents used most commonly, and heat is usually required (refer to section 1.2.3 for application of these reagents to prepare alkyl halides from alcohols).
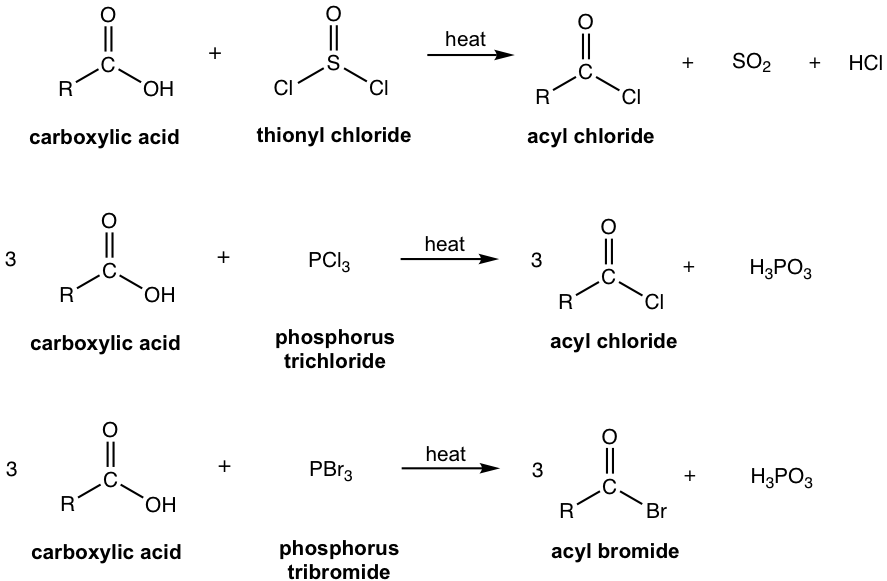
We will not go through the detailed mechanism for these reactions, however, they all involve nucleophilic addition-elimination process as well.
7.4.2 Preparations of Other Carboxylic Acid Derivatives From Acyl Halides
Since acyl halides are the most reactive, they are readily converted to other less reactive derivatives, as summarized in Fig. 7.4b below. The most effective way to synthesize anhydride, ester, and amide is by using acyl halide as a starting material, because of its high reactivity.
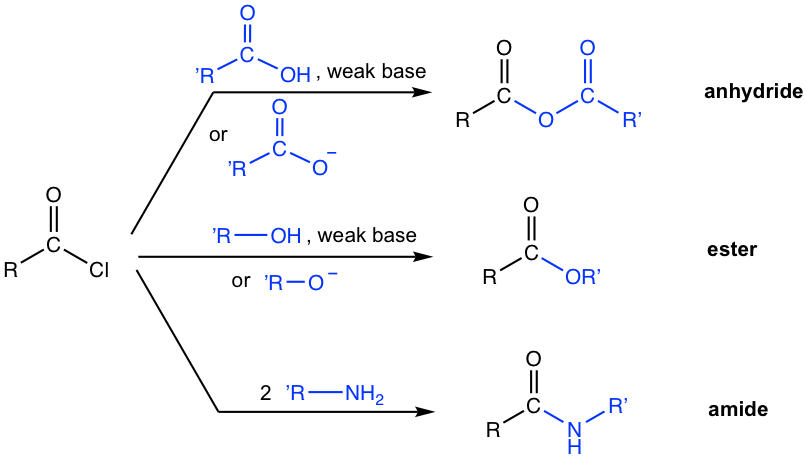
A couple of notes for the above reactions:
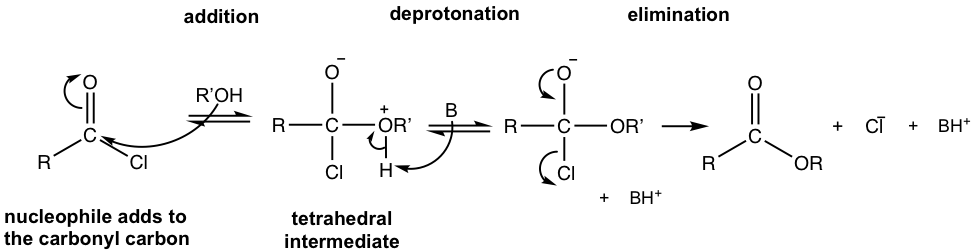
- Acyl chloride reacts with acid to produce anhydride, and acyl chloride reacts with alcohol to give ester. For these reactions, either a neutral reactant (RCOOH or ROH) or the salt of the reactant (RCOO– or RO–) can be used. When salt is used, i.e., with negatively charged nucleophiles, the mechanism follows the general mechanism in Fig. 7.3c exactly. When the neutral reactant is applied, a weak base is required to neutralize the by-product HCl and an extra deprotonation step is involved, the mechanism is shown below in Fig. 7.4c with synthesis of ester as the example.
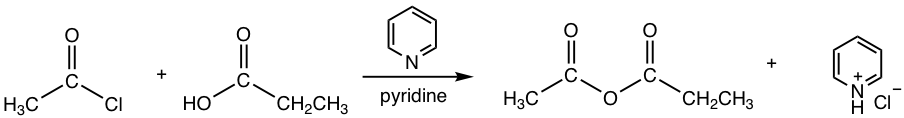
- When acyl chloride reacts with an amine, practically two equivalents of amine are required. The extra amount of amine is used to neutralize the by-product HCl, and ensure there will be enough unprotonated amine to react with the acyl halide. A specific example is given below.
7.4.3 Preparation of Carboxylic Acid Anhydrides
As mentioned in the previous section acyl chloride is a great starting compound to produce other derivatives, including anhydride. For the reaction between acyl chloride and an acid to prepare anhydride, amine (for example pyridine) is usually used as the weak base to neutralize the by-product HCl and drive the reaction to completion. Such a reaction is useful to prepare both symmetric anhydrides (R=R’) and mixed anhydrides (R≠R’).
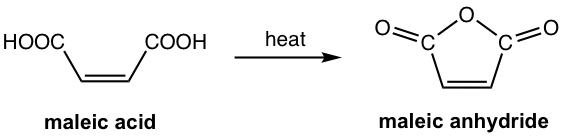
For dicarboxylic acid, intramolecular dehydration occurs between the two COOH groups by heating, and dicarboxylic acid is converted to cyclic anhydride. The reaction takes place readily when the cyclic anhydride is a five- or six-membered ring. The preparation of maleic anhydride is shown in Fig. 7.4e.
7.4.4 Preparations of Other Carboxylic Acid Derivatives From Anhydrides
Referring to the relative reactivity of carboxylic acid derivatives (section 7.3.3), we can predict that anhydride can be used to synthesize ester and amide, by reacting with alcohol or amine respectively, as shown in the equations below in Fig. 7.4f.
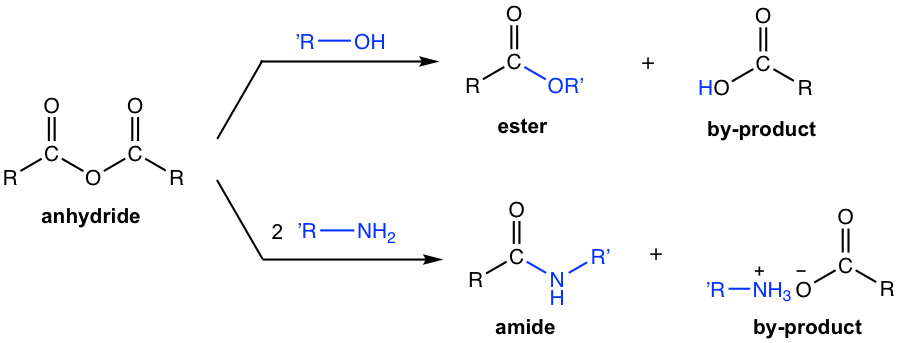
Please note that when anhydride is used for the synthesis of other derivatives, the organic by-product carboxylic acid (or the salt of carboxylic acid) is always generated.

